Cd Register Example . Published 30 june 2023 · last updated 22 january 2024 · see all updates. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Controlled drugs records in pharmacy. We have created a cd register template to use free of charge based around this guidance, a. Write “medicines for reuse” where you would usually write the person’s name. Name & address of supplier. Write the name strength and form. A) entries into the cd register i.
from www.studocu.com
Name & address of supplier. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Write “medicines for reuse” where you would usually write the person’s name. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. We have created a cd register template to use free of charge based around this guidance, a. Controlled drugs records in pharmacy. A) entries into the cd register i. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy.
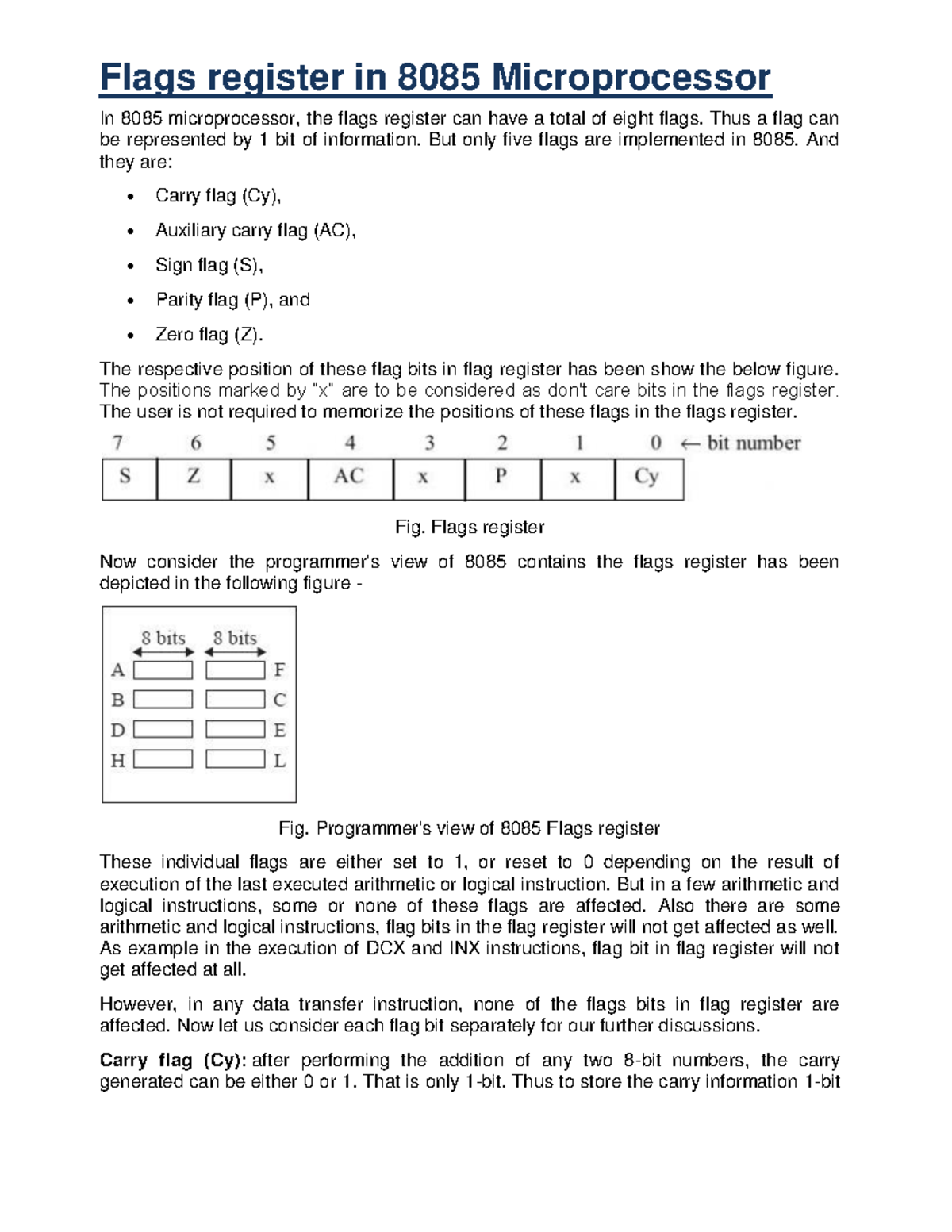
Flags register in 8085 Microprocessor Flags register in 8085
Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Name & address of supplier. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Controlled drugs records in pharmacy. Write “medicines for reuse” where you would usually write the person’s name. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Write the name strength and form. We have created a cd register template to use free of charge based around this guidance, a. Published 30 june 2023 · last updated 22 january 2024 · see all updates. A) entries into the cd register i.
From www.gadgetdealz.de
AZ Register für Bücher und CDSammlungen für 7,28 Euro inkl. Versand Cd Register Example Write the name strength and form. Name & address of supplier. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Published 30 june 2023 · last updated 22 january 2024 · see all updates. A) entries into the cd register i. Before using this register,. Cd Register Example.
From www.pdffiller.com
Fillable Online CDS Registration Fax Email Print pdfFiller Cd Register Example Name & address of supplier. Write the name strength and form. Published 30 june 2023 · last updated 22 january 2024 · see all updates. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Before using this register, a standard operating procedure (sop) should be. Cd Register Example.
From medicationtraining.co.uk
CD Register Medication Training Cd Register Example Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. We have created a cd register template to use free of charge based around this guidance, a.. Cd Register Example.
From www.youtube.com
Let's find out How does a CD work? YouTube Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. A) entries into the cd register i. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Controlled drugs records in pharmacy. Write the name strength and form. Controlled drugs (cd) register requirements are defined. Cd Register Example.
From www.nhstaysideadtc.scot.nhs.uk
1 Cd Register Example A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Before using this register, a standard operating procedure. Cd Register Example.
From www.slideserve.com
PPT Handling of Medication PowerPoint Presentation, free download Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend. Cd Register Example.
From www.this.nhs.uk
Firstoftype electronic controlled drugs register adds to hospital Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Write “medicines for reuse” where you would usually write the person’s name. A) entries into the cd register i. Controlled drugs (cd). Cd Register Example.
From csanalytical.com
Accreditations Cd Register Example Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. A) entries into the cd register i. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Name & address of supplier. Controlled drugs (cd) register requirements are. Cd Register Example.
From db-excel.com
Cd Ladder Excel Spreadsheet With Cd Ladder Spreadsheet Csserwis — db Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. Controlled drugs records in pharmacy. We have created a cd register template to use free of charge based around this guidance, a. Name & address of supplier. A) entries into the cd register i. Write “medicines for reuse” where you would usually write the person’s name.. Cd Register Example.
From itchol.com
CDS Application Form 2023, Check Steps to Apply Online (2022) Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. Controlled drugs records in pharmacy. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Before using this register, a standard operating procedure (sop) should be in place and signed. Cd Register Example.
From getclara.uk
Turbocharge your pharmacy — Clara Electronic CD Register and pharmacy Cd Register Example A) entries into the cd register i. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Write the name strength and form. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Before using this register, a standard operating procedure (sop) should be in place and. Cd Register Example.
From medicationtraining.co.uk
CD Register Medication Training Cd Register Example Controlled drugs records in pharmacy. A) entries into the cd register i. Write the name strength and form. Name & address of supplier. Write “medicines for reuse” where you would usually write the person’s name. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Controlled drugs (cd) register requirements are. Cd Register Example.
From www.templateroller.com
DA Form 4719 Fill Out, Sign Online and Download Fillable PDF Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. Write the name strength and form. Name & address of supplier. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. A) entries into the cd register i. Before using this register,. Cd Register Example.
From dl.dod.cyber.mil
DISN Connection Process Guide Cd Register Example Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Controlled drugs records in pharmacy. Write the name strength and form. We have created a cd register template to use free of charge based around this guidance, a. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Name & address of. Cd Register Example.
From www.etsy.com
PPE Register Template Etsy Cd Register Example Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Write “medicines for reuse” where you would usually write the person’s name. We have created a cd register template to use free of charge based around this. Cd Register Example.
From www.t3lshop.de
CDRegister + Etikketen mit genres Cd Register Example Controlled drugs records in pharmacy. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Write “medicines for reuse” where you would usually write the person’s name. A). Cd Register Example.
From www.lloydssupplies.co.uk
Record Keeping Cd Register Example A) entries into the cd register i. We have created a cd register template to use free of charge based around this guidance, a. Name & address of supplier. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Write the name strength and form. Write “medicines for reuse” where you would usually write the person’s. Cd Register Example.
From hubnet.io
Online Controlled Drugs Register Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. A controlled drugs register must be used to record details of. Cd Register Example.
From www.shiksha.com
How to fill CDS application form? Check stepbystep guide Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Controlled drugs (cd) register requirements are. Cd Register Example.
From njlabs.com
CDSLicense NJ Labs Cd Register Example Write the name strength and form. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Name & address of supplier. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Entries into the cd register, whether hard copy of electronic, must be. Cd Register Example.
From templatelab.com
39 Checkbook Register Templates [100 Free, Printable] ᐅ TemplateLab Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. Name & address of supplier. Write the name strength and form. Write “medicines for reuse” where you would usually write the person’s name. We have created a cd register template to use free of charge based around this guidance, a. Controlled drugs (cd) register requirements are. Cd Register Example.
From www.studocu.com
Flags register in 8085 Microprocessor Flags register in 8085 Cd Register Example A) entries into the cd register i. Write the name strength and form. Write “medicines for reuse” where you would usually write the person’s name. Name & address of supplier. Controlled drugs records in pharmacy. Published 30 june 2023 · last updated 22 january 2024 · see all updates. We have created a cd register template to use free of. Cd Register Example.
From jobschat.in
UPSC CDS 1 Notification 2021 345 UPSC CDS 1 Vacancy Cd Register Example A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend. Cd Register Example.
From getclara.uk
Turbocharge your pharmacy — Clara Electronic CD Register and pharmacy Cd Register Example Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. We have created a cd register template to use free of charge based around this guidance, a. Write the name strength and form.. Cd Register Example.
From www.youtube.com
CD Register and Address Descriptor. YouTube Cd Register Example Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Controlled drugs records in pharmacy. Write “medicines for reuse” where you would usually write the person’s name. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Before using this register, a standard operating procedure (sop) should. Cd Register Example.
From www.nhstaysideadtc.scot.nhs.uk
1 Cd Register Example Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. We have created a cd register template to use free of charge based around this guidance, a. Controlled drugs records in pharmacy. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Before using this register, a. Cd Register Example.
From www.nhstaysideadtc.scot.nhs.uk
1 Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. We have created a cd register. Cd Register Example.
From marcuskeong.com
How to Open a CDS Account in Malaysia with Bursa Anywhere Cd Register Example A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Write the name strength and form. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Entries into the cd register, whether hard copy of electronic, must be made as soon as. Cd Register Example.
From pharmcd.co.uk
PharmCD online CD register for UK pharmacies and dispensing doctors Cd Register Example Controlled drugs records in pharmacy. Write “medicines for reuse” where you would usually write the person’s name. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Write the name strength and form. Name & address of supplier. Before using this register, a standard operating procedure. Cd Register Example.
From www.valleynorthern.com
Controlled Drugs Register Book Valley Northern Cd Register Example Name & address of supplier. We have created a cd register template to use free of charge based around this guidance, a. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Entries into the cd register, whether hard copy of electronic, must be made as. Cd Register Example.
From www.shiksha.com
CDS Registration, Application Form 2021 How to Apply Online Cd Register Example Published 30 june 2023 · last updated 22 january 2024 · see all updates. Controlled drugs records in pharmacy. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Write “medicines for reuse” where you would usually write the person’s name. Entries into the cd register, whether hard copy of electronic, must be made as soon. Cd Register Example.
From www.shiksha.com
How to fill CDS application form? Check stepbystep guide Cd Register Example Write “medicines for reuse” where you would usually write the person’s name. A controlled drugs register must be used to record details of any schedule 1 and schedule 2 cds received or supplied by a pharmacy. Published 30 june 2023 · last updated 22 january 2024 · see all updates. Before using this register, a standard operating procedure (sop) should. Cd Register Example.
From vffow-buchverkauf.de
Wie der VFFOW digital wurde Die CDs erhalten ein neues Design Cd Register Example Name & address of supplier. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Controlled drugs records in pharmacy. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Write the name strength and form. Controlled drugs. Cd Register Example.
From www.pinterest.com
Free+Printable+Check+Registers+Template Checkbook register, Printable Cd Register Example We have created a cd register template to use free of charge based around this guidance, a. Controlled drugs (cd) register requirements are defined in uk law by the medicines act. Entries into the cd register, whether hard copy of electronic, must be made as soon as practicable, and in. Name & address of supplier. Write the name strength and. Cd Register Example.
From orders.ashtons.com
ASHTONS CONTROLLED DRUG REGISTER Ashtons Cd Register Example A) entries into the cd register i. We have created a cd register template to use free of charge based around this guidance, a. Before using this register, a standard operating procedure (sop) should be in place and signed by all suitably qualified personnel who intend to. Write “medicines for reuse” where you would usually write the person’s name. Controlled. Cd Register Example.