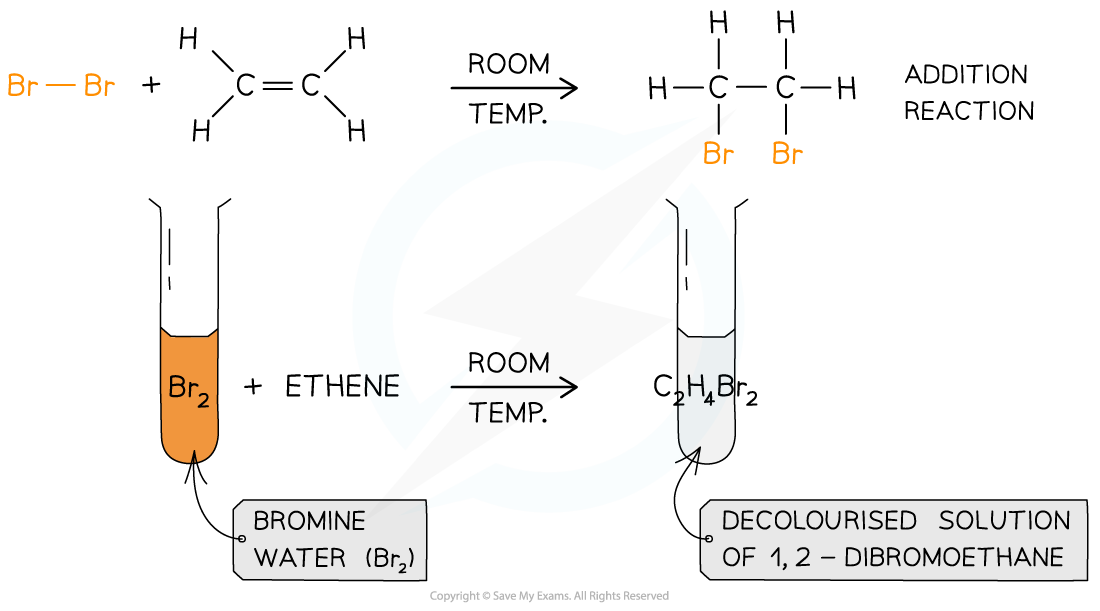
Bromine Water Gcse . a simple test with bromine water can be used to tell the difference between an alkane and an alkene. An alkene will turn brown. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. bromine water reacts with alkenes. Students should be able to recall the colour change when bromine water reacts with an alkene. — bromine is a diatomic molecule, which means it consists of two bromine atoms. bromine water is an orange solution of bromine. When we add bromine water to a compound containing a double bond, an addition reaction takes place. — we know from gcse that the test for an alkene or a c=c double bond is to. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. It becomes colourless when it is shaken with an alkene. We can use bromine water to test for alkenes. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes.
from www.linstitute.net
bromine water reacts with alkenes. An alkene will turn brown. It becomes colourless when it is shaken with an alkene. We can use bromine water to test for alkenes. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. bromine water is an orange solution of bromine. — we know from gcse that the test for an alkene or a c=c double bond is to. — bromine is a diatomic molecule, which means it consists of two bromine atoms.
CIE A Level Chemistry复习笔记3.2.9 Test for Unsaturation翰林国际教育
Bromine Water Gcse We can use bromine water to test for alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. When we add bromine water to a compound containing a double bond, an addition reaction takes place. Students should be able to recall the colour change when bromine water reacts with an alkene. It becomes colourless when it is shaken with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. bromine water is an orange solution of bromine. — we know from gcse that the test for an alkene or a c=c double bond is to. We can use bromine water to test for alkenes. An alkene will turn brown. bromine water reacts with alkenes. — bromine is a diatomic molecule, which means it consists of two bromine atoms. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene.
From exoiawwlq.blob.core.windows.net
Use Bromine Water Test at Bryan Green blog Bromine Water Gcse When we add bromine water to a compound containing a double bond, an addition reaction takes place. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. An alkene will turn brown. bromine. Bromine Water Gcse.
From www.sciencephoto.com
Bromine water test for alkenes Stock Image C029/0955 Science Photo Library Bromine Water Gcse An alkene will turn brown. It becomes colourless when it is shaken with an alkene. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. When we add bromine water to a compound containing. Bromine Water Gcse.
From slidetodoc.com
2012 Chemistry of SULPHUR Comprehensive tutorial notes POWERPOINT Bromine Water Gcse bromine water reacts with alkenes. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. bromine water is an orange solution of bromine. — bromine is a diatomic molecule, which means it consists of two bromine atoms. An alkene will turn brown. We can use bromine water to test for alkenes.. Bromine Water Gcse.
From www.toppr.com
How many moles of bromine water will be required for the complete bromination of 6 moles of phenol? Bromine Water Gcse bromine water reacts with alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. An alkene will turn brown. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. When we add bromine water to a compound containing a double bond, an. Bromine Water Gcse.
From www.youtube.com
Reactions with Bromine Water YouTube Bromine Water Gcse An alkene will turn brown. Students should be able to recall the colour change when bromine water reacts with an alkene. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. bromine water reacts with alkenes. We can use bromine water to test for alkenes. alkenes are more reactive than alkanes and. Bromine Water Gcse.
From www.numerade.com
SOLVED When butene is treated with liquid bromine under aqueous condition; what are the major Bromine Water Gcse — bromine is a diatomic molecule, which means it consists of two bromine atoms. An alkene will turn brown. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. bromine water reacts with alkenes. — we know from gcse that the test for an alkene or a. Bromine Water Gcse.
From byjus.com
Give a brief description of bromine water test. 5 marks Bromine Water Gcse It becomes colourless when it is shaken with an alkene. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. Students should be able to recall the colour change when bromine water reacts with an alkene. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. —. Bromine Water Gcse.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Water Gcse As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. — we know from gcse that the test for an alkene or a c=c double bond is to. An alkene will turn brown. It becomes colourless when it is shaken with an alkene. bromine water is an orange solution of bromine. . Bromine Water Gcse.
From www.youtube.com
Explainhy, ethene decolourises bromine water whereas ethane does not. YouTube Bromine Water Gcse alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. We can use bromine water to test for alkenes. When we add bromine water to a compound containing a double. Bromine Water Gcse.
From byjus.com
How do you test for an alkene? Bromine Water Gcse As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. We can use bromine water to test for alkenes. — bromine is a diatomic molecule, which means it consists of two bromine atoms.. Bromine Water Gcse.
From pixels.com
Bromine Water Test Photograph by Martyn F. Chillmaid/science Photo Library Pixels Bromine Water Gcse It becomes colourless when it is shaken with an alkene. bromine water reacts with alkenes. bromine water is an orange solution of bromine. When we add bromine water to a compound containing a double bond, an addition reaction takes place. a simple test with bromine water can be used to tell the difference between an alkane and. Bromine Water Gcse.
From www.savemyexams.co.uk
Bromine & Alkenes (4.4.2) Edexcel IGCSE Chemistry Double Science Revision Notes 2019 Save Bromine Water Gcse bromine water is an orange solution of bromine. Students should be able to recall the colour change when bromine water reacts with an alkene. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. When we add bromine water to a compound containing a double bond, an addition reaction takes place. —. Bromine Water Gcse.
From www.sciencephoto.com
Test tube of bromine water Stock Image C029/0952 Science Photo Library Bromine Water Gcse We can use bromine water to test for alkenes. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. When we add bromine water to a compound containing a double bond, an addition reaction takes place. a simple test with bromine water can be used to tell the difference between an alkane and. Bromine Water Gcse.
From www.thesciencehive.co.uk
Alkanes and Alkenes (GCSE) — the science sauce Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. It becomes colourless when it is shaken with an alkene. — bromine is a diatomic molecule, which means it consists of two bromine atoms. An alkene will turn brown. We can use bromine water to test for alkenes. You. Bromine Water Gcse.
From www.sciencephoto.com
Decolouring bromine water Stock Image A500/0532 Science Photo Library Bromine Water Gcse It becomes colourless when it is shaken with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. — bromine is a diatomic molecule, which means it consists of two bromine atoms. a simple test with bromine water can be used to tell the difference between. Bromine Water Gcse.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Water Gcse When we add bromine water to a compound containing a double bond, an addition reaction takes place. bromine water is an orange solution of bromine. An alkene will turn brown. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. a simple test with bromine water can be. Bromine Water Gcse.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Bromine Water Gcse — we know from gcse that the test for an alkene or a c=c double bond is to. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. bromine water reacts with alkenes. a simple test with bromine water can be used to tell the difference between an alkane and an. Bromine Water Gcse.
From studylib.net
Bromine Water Test Bromine Water Gcse bromine water is an orange solution of bromine. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. We can use bromine water to test for alkenes. Students should be able to recall the colour change when bromine water reacts with an alkene. — we know from gcse that the test for. Bromine Water Gcse.
From pixels.com
Test Tube Of Bromine Water Photograph by Martyn F. Chillmaid/science Photo Library Pixels Bromine Water Gcse An alkene will turn brown. — bromine is a diatomic molecule, which means it consists of two bromine atoms. bromine water is an orange solution of bromine. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. — we know from gcse that the test for an alkene or a c=c. Bromine Water Gcse.
From scienceready.com.au
Bromine Water Test Science Ready Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. Students should be able to recall the colour change when bromine water reacts with an alkene. — we know from gcse that the test for an alkene or a c=c double bond is to. It becomes colourless when it. Bromine Water Gcse.
From www.youtube.com
GCSE Chemistry Bromine Water Test YouTube Bromine Water Gcse bromine water is an orange solution of bromine. An alkene will turn brown. — we know from gcse that the test for an alkene or a c=c double bond is to. Students should be able to recall the colour change when bromine water reacts with an alkene. You can use bromine water, which is an orange solution, to. Bromine Water Gcse.
From www.youtube.com
Identifying Alkenes with Bromine Water YouTube Bromine Water Gcse An alkene will turn brown. — bromine is a diatomic molecule, which means it consists of two bromine atoms. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes.. Bromine Water Gcse.
From www.youtube.com
Experiment of Bromine Water Test🔥 Organic Chemistry Imran Sir Vibrant Academy YouTube Bromine Water Gcse bromine water is an orange solution of bromine. Students should be able to recall the colour change when bromine water reacts with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. We can use bromine water to test for alkenes. When we add bromine water to. Bromine Water Gcse.
From www.doubtnut.com
Explain the action of bromine water on phenol (carbolic acid). Bromine Water Gcse As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. — we know. Bromine Water Gcse.
From www.youtube.com
How to Prepare BROMINE WATER in the Lab IGCSE Science Lab YouTube Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. bromine water is an orange solution of bromine. It becomes colourless when it is shaken with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. . Bromine Water Gcse.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine Water Gcse bromine water is an orange solution of bromine. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. It becomes colourless when it is shaken with an alkene. — we know from gcse that the test for an alkene or a c=c double bond is to. We can use bromine water to. Bromine Water Gcse.
From www.coursehero.com
Bromine water (golden yellow solution) and the purple potassium... Course Hero Bromine Water Gcse It becomes colourless when it is shaken with an alkene. — we know from gcse that the test for an alkene or a c=c double bond is to. bromine water is an orange solution of bromine. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. alkenes are more reactive than. Bromine Water Gcse.
From www.docsity.com
Bromine extraction GCSE Schemes and Mind Maps Chemistry Docsity Bromine Water Gcse It becomes colourless when it is shaken with an alkene. bromine water is an orange solution of bromine. — bromine is a diatomic molecule, which means it consists of two bromine atoms. When we add bromine water to a compound containing a double bond, an addition reaction takes place. a simple test with bromine water can be. Bromine Water Gcse.
From understandingstem.com
Bromine water test How to differentiate between alkanes and alkenes experimentally Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. An alkene will turn brown. — bromine is a diatomic molecule, which means it consists of two bromine atoms. As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. bromine water is. Bromine Water Gcse.
From www.coursehero.com
[Solved] what happen if you combine 1 ml bromine water and few drops... Course Hero Bromine Water Gcse — bromine is a diatomic molecule, which means it consists of two bromine atoms. a simple test with bromine water can be used to tell the difference between an alkane and an alkene. — we know from gcse that the test for an alkene or a c=c double bond is to. bromine water is an orange. Bromine Water Gcse.
From www.bbc.co.uk
What is a displacement reaction? BBC Bitesize Bromine Water Gcse As we’ve just seen, bromine water undergoes a colour change when it reacts with an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. An alkene will turn brown. Students should be able to recall the colour change when bromine water reacts with an alkene. bromine water. Bromine Water Gcse.
From www.youtube.com
Bromine Water Experiment YouTube Bromine Water Gcse alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. It becomes colourless when it is shaken with an alkene. When we add bromine water to a compound containing a double bond, an addition reaction takes place. Students should be able to recall the colour change when bromine water reacts. Bromine Water Gcse.
From dxonpkucv.blob.core.windows.net
Bromine Water Acid Or Base at Derek Jones blog Bromine Water Gcse We can use bromine water to test for alkenes. It becomes colourless when it is shaken with an alkene. When we add bromine water to a compound containing a double bond, an addition reaction takes place. Students should be able to recall the colour change when bromine water reacts with an alkene. alkenes are more reactive than alkanes and. Bromine Water Gcse.
From www.linstitute.net
CIE A Level Chemistry复习笔记3.2.9 Test for Unsaturation翰林国际教育 Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. We can use bromine water to test for alkenes. You can use bromine water, which is an orange solution, to distinguish between alkanes and alkenes. It becomes colourless when it is shaken with an alkene. — bromine is a. Bromine Water Gcse.
From www.pinterest.com
Saturated and unsaturated oils in bromine water. Bromine Water Gcse a simple test with bromine water can be used to tell the difference between an alkane and an alkene. alkenes are more reactive than alkanes and react with bromine water, which is used as a test for alkenes. It becomes colourless when it is shaken with an alkene. bromine water reacts with alkenes. bromine water is. Bromine Water Gcse.