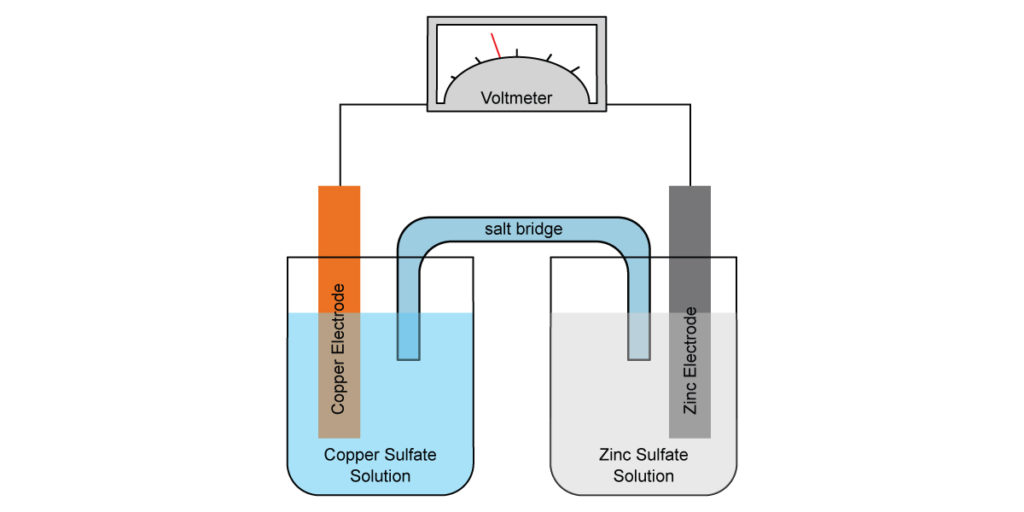
How Does Electrochemical Cell Work . What would you put inside a salt bridge? how electrochemical cells work. Electrochemical cells consist of two electrodes (conducting materials that serve. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. What processes are responsible for conduction of electricity in an electrochemical cell? This kind of cell includes the galvanic, or. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. describe what you know about an electrochemical cell. Describe two types of situations that would There are two types of
from theedge.com.hk
describe what you know about an electrochemical cell. This kind of cell includes the galvanic, or. What would you put inside a salt bridge? What processes are responsible for conduction of electricity in an electrochemical cell? There are two types of Describe two types of situations that would an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Electrochemical cells consist of two electrodes (conducting materials that serve. how electrochemical cells work.
Chemistry How To Electrochemical Cell The Edge
How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What would you put inside a salt bridge? This kind of cell includes the galvanic, or. describe what you know about an electrochemical cell. how electrochemical cells work. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Describe two types of situations that would There are two types of an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are responsible for conduction of electricity in an electrochemical cell? Electrochemical cells consist of two electrodes (conducting materials that serve.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell How Does Electrochemical Cell Work What would you put inside a salt bridge? Electrochemical cells consist of two electrodes (conducting materials that serve. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are. How Does Electrochemical Cell Work.
From stoplearn.com
Electrochemical Cells 2023 How Does Electrochemical Cell Work an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. describe what you know about an electrochemical cell. Electrochemical cells consist of two electrodes (conducting materials that serve. Describe two types of situations that would What would you put. How Does Electrochemical Cell Work.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge How Does Electrochemical Cell Work describe what you know about an electrochemical cell. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What. How Does Electrochemical Cell Work.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii How Does Electrochemical Cell Work how electrochemical cells work. This kind of cell includes the galvanic, or. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What would you put inside a salt bridge? There are two types of in an electrochemical cell, we allow electrons to be transferred, which will spontaneously. How Does Electrochemical Cell Work.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells How Does Electrochemical Cell Work This kind of cell includes the galvanic, or. Electrochemical cells consist of two electrodes (conducting materials that serve. What processes are responsible for conduction of electricity in an electrochemical cell? describe what you know about an electrochemical cell. What would you put inside a salt bridge? an apparatus that is used to generate electricity from a spontaneous redox. How Does Electrochemical Cell Work.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) How Does Electrochemical Cell Work in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. how electrochemical cells work. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. There are two types of describe what you know about an electrochemical cell. an. How Does Electrochemical Cell Work.
From www.alamy.com
Basic components of an electrochemical cell Stock Photo Alamy How Does Electrochemical Cell Work This kind of cell includes the galvanic, or. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. how electrochemical. How Does Electrochemical Cell Work.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell How Does Electrochemical Cell Work how electrochemical cells work. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. There are two types of What would you put inside a salt bridge? an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. How Does Electrochemical Cell Work.
From mavink.com
Electrochemical Cell Diagram How Does Electrochemical Cell Work There are two types of how electrochemical cells work. Electrochemical cells consist of two electrodes (conducting materials that serve. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Describe two types of situations that would What would you. How Does Electrochemical Cell Work.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical cells consist of two electrodes (conducting materials that serve. how electrochemical cells work. What processes are responsible for conduction of electricity in an electrochemical cell? in an electrochemical cell, we allow electrons to be transferred, which will. How Does Electrochemical Cell Work.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses How Does Electrochemical Cell Work This kind of cell includes the galvanic, or. Describe two types of situations that would Electrochemical cells consist of two electrodes (conducting materials that serve. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. What would you put inside. How Does Electrochemical Cell Work.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis How Does Electrochemical Cell Work Describe two types of situations that would This kind of cell includes the galvanic, or. There are two types of an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are responsible for conduction of electricity in an electrochemical cell? in an electrochemical cell, we allow electrons. How Does Electrochemical Cell Work.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts How Does Electrochemical Cell Work There are two types of Electrochemical cells consist of two electrodes (conducting materials that serve. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe two types of situations that would What would you put inside a salt bridge? This kind of cell includes the galvanic, or. What processes. How Does Electrochemical Cell Work.
From www.youtube.com
How does a battery work? Electrochemical cells, redox, MgCu cell in How Does Electrochemical Cell Work in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? This kind of cell includes the galvanic, or. What processes are responsible for conduction of electricity in an electrochemical cell? an electrochemical cell is a device that produces an electric current from. How Does Electrochemical Cell Work.
From cemfmfxu.blob.core.windows.net
Electrochemical Cell Used For at Daniel Byrne blog How Does Electrochemical Cell Work an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Electrochemical cells consist of two electrodes (conducting materials that serve. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.. How Does Electrochemical Cell Work.
From www.pinterest.com
Electrochemical Cells Chemwiki Chemistry Textbook, Gcse Chemistry How Does Electrochemical Cell Work There are two types of What processes are responsible for conduction of electricity in an electrochemical cell? in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. This kind of cell includes the galvanic, or. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. How Does Electrochemical Cell Work.
From mavink.com
Electrochemical Cell Diagram How Does Electrochemical Cell Work This kind of cell includes the galvanic, or. describe what you know about an electrochemical cell. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Describe two types of situations that would What would you put inside a salt bridge? how electrochemical cells work. an apparatus that. How Does Electrochemical Cell Work.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. how electrochemical cells work. There are two types of This kind of cell includes the galvanic, or. Describe two types of situations that would Electrochemical cells consist of two electrodes (conducting materials that serve. What would you put inside. How Does Electrochemical Cell Work.
From www.youtube.com
Class 12 How electricity is generated in an electrochemical cell YouTube How Does Electrochemical Cell Work What would you put inside a salt bridge? describe what you know about an electrochemical cell. how electrochemical cells work. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction. How Does Electrochemical Cell Work.
From www.researchgate.net
Electrochemical cell. Download Scientific Diagram How Does Electrochemical Cell Work There are two types of What would you put inside a salt bridge? Describe two types of situations that would Electrochemical cells consist of two electrodes (conducting materials that serve. describe what you know about an electrochemical cell. how electrochemical cells work. This kind of cell includes the galvanic, or. an electrochemical cell is a device that. How Does Electrochemical Cell Work.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or. There are two types of how electrochemical cells work. Electrochemical cells consist of two electrodes (conducting materials that serve. an apparatus that is used to generate electricity from a spontaneous. How Does Electrochemical Cell Work.
From theedge.com.hk
Chemistry How To Electrochemical Cell The Edge How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. describe what you know about an electrochemical cell. What would you put inside a salt bridge? There are two types of Describe two types of situations that would how electrochemical cells work. What processes are responsible for conduction. How Does Electrochemical Cell Work.
From mmerevise.co.uk
Electrochemical Cells MME How Does Electrochemical Cell Work Electrochemical cells consist of two electrodes (conducting materials that serve. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. how electrochemical cells work. What processes are responsible for conduction of electricity in an electrochemical cell? in an. How Does Electrochemical Cell Work.
From circuitengineshavie88.z5.web.core.windows.net
Electrochemical Cell Diagram How Does Electrochemical Cell Work an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. Electrochemical cells consist of two electrodes (conducting materials that serve. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.. How Does Electrochemical Cell Work.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME How Does Electrochemical Cell Work What processes are responsible for conduction of electricity in an electrochemical cell? This kind of cell includes the galvanic, or. What would you put inside a salt bridge? in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. There are two types of describe what you know about an electrochemical. How Does Electrochemical Cell Work.
From www.clutchprep.com
Electrochemical Cells Analytical Chemistry Video Clutch Prep How Does Electrochemical Cell Work What processes are responsible for conduction of electricity in an electrochemical cell? an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Electrochemical cells consist of two electrodes (conducting materials that serve. There are two types of how electrochemical cells work. an apparatus that is used to generate. How Does Electrochemical Cell Work.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Describe two types of situations that would an apparatus that is used to generate electricity from a spontaneous redox reaction. How Does Electrochemical Cell Work.
From learningschoolletopisaln.z22.web.core.windows.net
Schematic Of Electrochemical Cells How Does Electrochemical Cell Work in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Electrochemical cells consist of two electrodes (conducting materials that serve. What would you put inside a salt bridge? There are two types of an electrochemical cell is a device that produces an electric current from energy released by a spontaneous. How Does Electrochemical Cell Work.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses How Does Electrochemical Cell Work in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? This kind of cell includes the galvanic, or. There are two types of an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. How Does Electrochemical Cell Work.
From www.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What processes are responsible for conduction of electricity in an electrochemical cell? What would you put inside a salt bridge? how electrochemical cells work. Electrochemical cells consist of two electrodes (conducting materials that serve. There are two types of. How Does Electrochemical Cell Work.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types How Does Electrochemical Cell Work an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. how electrochemical cells work. What would you put inside a salt bridge? in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. This kind of cell includes the galvanic, or.. How Does Electrochemical Cell Work.
From www.researchgate.net
Schematic diagram of the electrochemical cell used in this work How Does Electrochemical Cell Work There are two types of Describe two types of situations that would an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? Electrochemical. How Does Electrochemical Cell Work.
From learningschoolletopisaln.z22.web.core.windows.net
Electrochemical Cells In Chemistry How Does Electrochemical Cell Work an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell. an electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. What would you put inside a salt bridge? What processes. How Does Electrochemical Cell Work.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field How Does Electrochemical Cell Work This kind of cell includes the galvanic, or. There are two types of how electrochemical cells work. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. How Does Electrochemical Cell Work.
From www.researchgate.net
(A) Schematic diagram of the electrochemical cell for converting CaCO3 How Does Electrochemical Cell Work What would you put inside a salt bridge? There are two types of Electrochemical cells consist of two electrodes (conducting materials that serve. This kind of cell includes the galvanic, or. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical. How Does Electrochemical Cell Work.