Indicator Used In Iodometric Titration . In an iodometric titration, which indicator is used? Starch is typically used as the indication for iodine titrations. iodometric methods of analysis have a wide applicability for the following reasons: The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To know when the reaction is complete, we use starch solution as an indicator. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. iodometric titration can be of two types 1. Potassium iodide, ki, is readily available in high purity.
from slidetodoc.com
A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric methods of analysis have a wide applicability for the following reasons: iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titration can be of two types 1. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. Starch is typically used as the indication for iodine titrations. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. In an iodometric titration, which indicator is used? iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,.
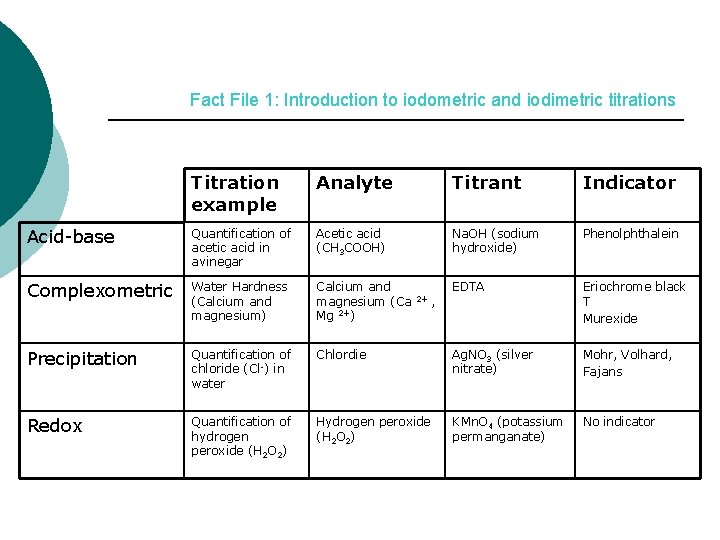
Fact File 1 INTRODUCTION TO IODOMETRIC AND IODIMETRIC
Indicator Used In Iodometric Titration thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. iodometric titration can be of two types 1. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Potassium iodide, ki, is readily available in high purity. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. In an iodometric titration, which indicator is used? A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. To know when the reaction is complete, we use starch solution as an indicator. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. Starch is typically used as the indication for iodine titrations. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric methods of analysis have a wide applicability for the following reasons:
From celkpusg.blob.core.windows.net
What Is Iodometric Titration And Iodimetric Titration at Joseph Leach blog Indicator Used In Iodometric Titration In an iodometric titration, which indicator is used? It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric methods of analysis have a wide applicability for the following reasons: To know when the reaction. Indicator Used In Iodometric Titration.
From cermxony.blob.core.windows.net
Titration Indicator Role at Jared Marquis blog Indicator Used In Iodometric Titration In an iodometric titration, which indicator is used? It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. iodometric titration can be of two types 1. A good indicator, starch, is available to signal the equivalence. Indicator Used In Iodometric Titration.
From www.youtube.com
Dissolved oxygen ( DO) of water sample Winkler's iodometric Titration Indicator Used In Iodometric Titration thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. In an iodometric titration, which indicator is used? Starch is typically used as the indication for iodine titrations. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The direct iodometric titration method. Indicator Used In Iodometric Titration.
From celkpusg.blob.core.windows.net
What Is Iodometric Titration And Iodimetric Titration at Joseph Leach blog Indicator Used In Iodometric Titration iodometric titration can be of two types 1. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. To know when the reaction is complete, we use starch solution as an indicator. iodometric methods of analysis have a. Indicator Used In Iodometric Titration.
From www.toppr.com
Selection the correct statements.1. The reaction belongs to iodometric Indicator Used In Iodometric Titration In an iodometric titration, which indicator is used? iodometric titration can be of two types 1. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. To know when the reaction is complete, we use starch solution as an indicator. thanks to its relatively low, ph independent redox potential, and reversibility of. Indicator Used In Iodometric Titration.
From dwzymndbeco.blob.core.windows.net
Iodometric Titration End Point Detection at Joseph Monger blog Indicator Used In Iodometric Titration Starch is typically used as the indication for iodine titrations. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric titration. Indicator Used In Iodometric Titration.
From www.youtube.com
IODOMETRIC TITRATION REDOX TITRATION YouTube Indicator Used In Iodometric Titration Starch is typically used as the indication for iodine titrations. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. thanks to its relatively. Indicator Used In Iodometric Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator Used In Iodometric Titration iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. iodometric methods of analysis have a wide applicability for the following reasons: iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Potassium iodide, ki, is readily available in high purity. iodometric titration. Indicator Used In Iodometric Titration.
From www.youtube.com
Estimation of Cu2+ ions by Iodometric Titration Method Iodometric Indicator Used In Iodometric Titration iodometric titration can be of two types 1. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. Starch is typically used as the. Indicator Used In Iodometric Titration.
From www.numerade.com
SOLVED What are the differences between iodometric and iodimetric Indicator Used In Iodometric Titration Potassium iodide, ki, is readily available in high purity. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric methods of analysis have a wide applicability for the following reasons: In an iodometric titration, which indicator is used? iodometric titration can be of two types 1. iodometric titrations are particularly useful in the analysis. Indicator Used In Iodometric Titration.
From www.doubtnut.com
In Iodometric titration which indicator is used to detect end point of Indicator Used In Iodometric Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. To know when the reaction is complete,. Indicator Used In Iodometric Titration.
From vinipul.com
Iodometric Applications Indicator Used In Iodometric Titration iodometric titration can be of two types 1. In an iodometric titration, which indicator is used? The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. To know when the reaction is complete, we use starch solution as an. Indicator Used In Iodometric Titration.
From cegtwwll.blob.core.windows.net
Indicator Work In A Titration at Amy Tanner blog Indicator Used In Iodometric Titration iodometric titration can be of two types 1. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. In an iodometric titration, which indicator is used? Potassium iodide, ki, is readily available in high purity. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released.. Indicator Used In Iodometric Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Indicator Used In Iodometric Titration iodometric methods of analysis have a wide applicability for the following reasons: thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. In an iodometric titration, which indicator is used? iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. It works by. Indicator Used In Iodometric Titration.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Indicator Used In Iodometric Titration iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. To know when the reaction is complete,. Indicator Used In Iodometric Titration.
From www.youtube.com
Iodometric Titration of Cu(II ) ions against 0.01M Sodium Thiosulphate Indicator Used In Iodometric Titration A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. In an iodometric titration, which indicator is used? The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titration can be of two types 1. Potassium iodide, ki, is readily available in high purity. iodometric titration is. Indicator Used In Iodometric Titration.
From www.gbu-presnenskij.ru
Iodometric Titration Principle, Example, Advantages, 47 OFF Indicator Used In Iodometric Titration iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. Potassium iodide, ki, is readily available in high purity. In an iodometric titration, which indicator is used? To know when the reaction is complete, we use starch solution as an indicator. iodometric methods of analysis have a wide applicability for the. Indicator Used In Iodometric Titration.
From www.numerade.com
SOLVED(A) Starch is the indicator used in the iodometric titrations Indicator Used In Iodometric Titration The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. iodometric titration can be of two types 1. iodometric. Indicator Used In Iodometric Titration.
From cedpcfbl.blob.core.windows.net
Titration Indicator Analysis at Donna Kendall blog Indicator Used In Iodometric Titration Potassium iodide, ki, is readily available in high purity. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate.. Indicator Used In Iodometric Titration.
From www.science-revision.co.uk
Titrations Indicator Used In Iodometric Titration Potassium iodide, ki, is readily available in high purity. iodometric methods of analysis have a wide applicability for the following reasons: In an iodometric titration, which indicator is used? iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. thanks to its relatively low, ph independent redox potential, and reversibility of. Indicator Used In Iodometric Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicator Used In Iodometric Titration iodometric titration can be of two types 1. Starch is typically used as the indication for iodine titrations. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. In an iodometric titration, which indicator is used? To know when the reaction is complete, we use starch solution as an indicator. iodometric titrations are particularly useful in. Indicator Used In Iodometric Titration.
From dwzymndbeco.blob.core.windows.net
Iodometric Titration End Point Detection at Joseph Monger blog Indicator Used In Iodometric Titration It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. iodometric methods of analysis have a wide applicability for the following reasons: A good indicator, starch, is available to signal the equivalence point in the. Indicator Used In Iodometric Titration.
From cegtwwll.blob.core.windows.net
Indicator Work In A Titration at Amy Tanner blog Indicator Used In Iodometric Titration thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. Potassium iodide, ki, is readily available in high purity. Starch is typically used as the indication for iodine titrations. iodometric methods of analysis have a wide applicability for the following reasons: iodometric titration is a method used to measure. Indicator Used In Iodometric Titration.
From gbu-taganskij.ru
Iodometric Titration Principle, Example, Advantages, 44 OFF Indicator Used In Iodometric Titration iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titration can be of two types 1. To know when the. Indicator Used In Iodometric Titration.
From www.studypool.com
SOLUTION Exp 5 Copper by iodometric titration Studypool Indicator Used In Iodometric Titration In an iodometric titration, which indicator is used? iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. Potassium iodide, ki, is readily available in high purity. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. A good indicator, starch, is available to signal the equivalence point in the reaction. Indicator Used In Iodometric Titration.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Indicator Used In Iodometric Titration thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. iodometric methods of analysis have a wide applicability for the following reasons: A good indicator, starch, is available to signal the equivalence point in the reaction between iodine and thiosulfate. iodometric titration can be of two types 1. . Indicator Used In Iodometric Titration.
From cegtwwll.blob.core.windows.net
Indicator Work In A Titration at Amy Tanner blog Indicator Used In Iodometric Titration To know when the reaction is complete, we use starch solution as an indicator. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. iodometric methods of analysis have a wide applicability for the following reasons: Starch is typically used as the indication for iodine titrations. Potassium iodide, ki, is. Indicator Used In Iodometric Titration.
From cejhmzmp.blob.core.windows.net
Why We Use Indicator In Titration at Pamela Hines blog Indicator Used In Iodometric Titration iodometric methods of analysis have a wide applicability for the following reasons: iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. To know when the reaction is complete, we use starch solution as an indicator. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide. Indicator Used In Iodometric Titration.
From gbu-taganskij.ru
Iodometric Titration Principle, Example, Advantages, 44 OFF Indicator Used In Iodometric Titration iodometric titration can be of two types 1. Starch is typically used as the indication for iodine titrations. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. Potassium iodide, ki, is readily available in high purity. thanks to. Indicator Used In Iodometric Titration.
From hyprowira.com
Iodometric Titration Functions and How It Works Indicator Used In Iodometric Titration iodometric titration can be of two types 1. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. Starch is typically used as the indication for iodine titrations. In an iodometric titration, which indicator is used?. Indicator Used In Iodometric Titration.
From www.researchgate.net
(a) Iodometric titration to determine stoichiometry of ACTU and BrO 3 Indicator Used In Iodometric Titration iodometric titration is a method used to measure the amount of an oxidizing agent in a solution. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. iodometric titration can be of. Indicator Used In Iodometric Titration.
From www.researchgate.net
The scheme of the equipment used for iodometric titration 1 , 2 Indicator Used In Iodometric Titration In an iodometric titration, which indicator is used? To know when the reaction is complete, we use starch solution as an indicator. iodometric titration can be of two types 1. thanks to its relatively low, ph independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be. It works by mixing the oxidizing agent with iodide ions,. Indicator Used In Iodometric Titration.
From www.youtube.com
Iodimetric Titration!!! iodimetric iodometric titration YouTube Indicator Used In Iodometric Titration Starch is typically used as the indication for iodine titrations. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. In an iodometric titration, which indicator is used? It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. thanks to its relatively low, ph independent redox. Indicator Used In Iodometric Titration.
From slidetodoc.com
Fact File 1 INTRODUCTION TO IODOMETRIC AND IODIMETRIC Indicator Used In Iodometric Titration The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. To know when the reaction is complete, we use starch solution as an indicator. iodometric titrations are particularly useful in the analysis of oxidizing agents such as chlorine, hydrogen peroxide,. iodometric titration can be of two types 1. iodometric titration is a method used to. Indicator Used In Iodometric Titration.
From www.fishersci.ca
Starch Indicator, 1 (w/v), Mercury Free, for Iodometric Titrations Indicator Used In Iodometric Titration To know when the reaction is complete, we use starch solution as an indicator. It works by mixing the oxidizing agent with iodide ions, which causes iodine to be released. iodometric titration can be of two types 1. The direct iodometric titration method (sometimes termed iodimetry) refers to titrations of. iodometric titration is a method used to measure. Indicator Used In Iodometric Titration.