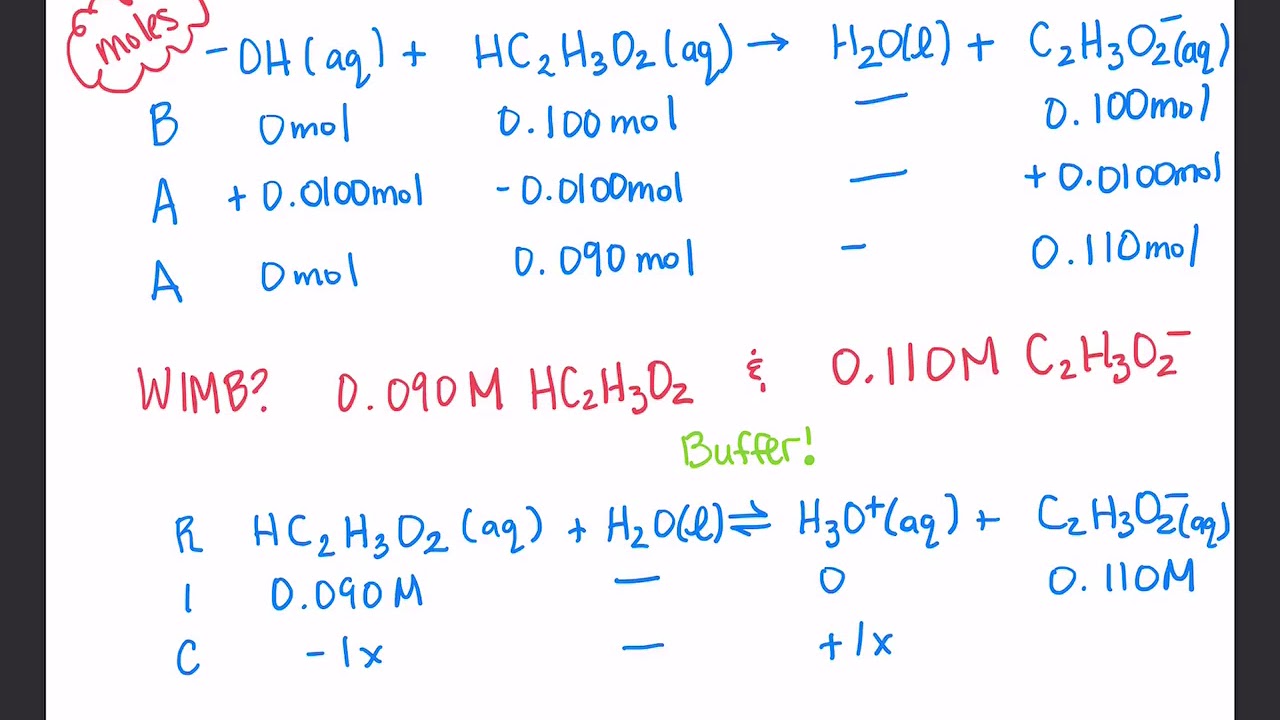
Ph Buffer Questions . A weak acid and it’s conjugate base in equilibrium: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. practice calculating the ph of a buffer with practice problems and explanations. this page describes simple acidic and alkaline buffer solutions and explains how they work. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. What is a buffer solution? It resists any change in the ph upon the addition of acidic or basic. A buffer solution is a mixture of a weak acid and its conjugate base. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Definition a buffer solution is one which. ph buffers the basic idea:
from www.youtube.com
this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? practice calculating the ph of a buffer with practice problems and explanations. It resists any change in the ph upon the addition of acidic or basic. ph buffers the basic idea: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. A weak acid and it’s conjugate base in equilibrium: Definition a buffer solution is one which. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a.
Calculating pH changes to a buffer solution YouTube
Ph Buffer Questions Definition a buffer solution is one which. What is a buffer solution? in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. It resists any change in the ph upon the addition of acidic or basic. this page describes simple acidic and alkaline buffer solutions and explains how they work. Definition a buffer solution is one which. ph buffers the basic idea: A buffer solution is a mixture of a weak acid and its conjugate base. A weak acid and it’s conjugate base in equilibrium: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. practice calculating the ph of a buffer with practice problems and explanations. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. Definition a buffer solution is one which. ph buffers the basic idea: practice calculating the ph of a buffer with practice problems and explanations. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of. Ph Buffer Questions.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube Ph Buffer Questions ph buffers the basic idea: What is a buffer solution? this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. It resists any change in the ph upon the addition of acidic or basic. in this section, we will see how to perform calculations to predict the. Ph Buffer Questions.
From byjus.com
22 Find the change in pH value of buffer solution when 50mL M/10 HCL is Ph Buffer Questions A buffer solution is a mixture of a weak acid and its conjugate base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. Definition a buffer solution is one which. practice calculating the ph of a buffer with practice problems and. Ph Buffer Questions.
From www.chegg.com
Solved pH Table 1. Data Table for buffers and samples for pH Ph Buffer Questions A weak acid and it’s conjugate base in equilibrium: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. Definition a buffer solution is one which. What is a buffer solution? It resists any change in the ph upon the addition of acidic or basic. A buffer solution is. Ph Buffer Questions.
From www.chegg.com
Solved Table 4 Buffer Solutions and pH Readings for Beakers Ph Buffer Questions A buffer solution is a mixture of a weak acid and its conjugate base. ph buffers the basic idea: Definition a buffer solution is one which. What is a buffer solution? this page describes simple acidic and alkaline buffer solutions and explains how they work. A weak acid and it’s conjugate base in equilibrium: the ability of. Ph Buffer Questions.
From www.numerade.com
⏩SOLVEDCalculate the pH of a buffer solution prepared by mixing 75 Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. A buffer solution is a mixture of a weak acid and its conjugate base. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. in this section, we will see how to perform calculations. Ph Buffer Questions.
From www.youtube.com
how to prepare a buffer with a particular pH YouTube Ph Buffer Questions What is a buffer solution? ph buffers the basic idea: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. practice calculating the ph of a buffer with practice problems and explanations. It resists any change in the ph upon the addition of acidic or basic. . Ph Buffer Questions.
From www.youtube.com
Calculating pH changes to a buffer solution YouTube Ph Buffer Questions ph buffers the basic idea: Definition a buffer solution is one which. A weak acid and it’s conjugate base in equilibrium: practice calculating the ph of a buffer with practice problems and explanations. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. this page describes. Ph Buffer Questions.
From chemguru.sg
Calculate pH of Buffer Solution Ph Buffer Questions A buffer solution is a mixture of a weak acid and its conjugate base. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid. Ph Buffer Questions.
From www.slideshare.net
Ph and buffer Ph Buffer Questions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. It resists any change in the ph upon the addition of acidic or basic. A weak acid and it’s conjugate base in equilibrium: A buffer solution is a mixture of a weak acid. Ph Buffer Questions.
From www.youtube.com
Calculating the pH of a buffer YouTube Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. A buffer solution is a mixture of a weak acid and its conjugate base. ph buffers the basic idea: What is a buffer solution? practice calculating the ph of a buffer with practice problems and explanations. this page describes simple acidic and alkaline. Ph Buffer Questions.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube Ph Buffer Questions Definition a buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution is a mixture of a weak acid and its conjugate base. practice calculating the ph of a buffer with practice problems and explanations. in this section, we will. Ph Buffer Questions.
From www.youtube.com
pH of Buffer Solution (Example) YouTube Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. this page describes simple acidic and alkaline buffer solutions and explains how they work. It resists any change in the ph upon the addition of acidic or basic. A weak acid and it’s conjugate base in. Ph Buffer Questions.
From www.bartleby.com
Answered (a) Calculate the pH of a buffer that… bartleby Ph Buffer Questions ph buffers the basic idea: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A weak acid and. Ph Buffer Questions.
From www.studocu.com
Chapter 11 Buffers practice questions ( solutions) 636 pharmaceutics Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Definition a buffer solution is one which. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. practice calculating the ph of a buffer with. Ph Buffer Questions.
From www.scribd.com
buffer questions PDF Ph Buffer Solution Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a. Ph Buffer Questions.
From www.youtube.com
How to Calculate PH of Buffer Solution YouTube Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. ph buffers the basic idea: A buffer solution is a mixture of a weak acid and its conjugate base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or. Ph Buffer Questions.
From kunduz.com
[ANSWERED] A pH buffer is a solution that resists ch... Physical Ph Buffer Questions ph buffers the basic idea: A weak acid and it’s conjugate base in equilibrium: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. practice calculating the ph of a buffer with practice problems and explanations. the ability of a buffer solution to resist large changes. Ph Buffer Questions.
From www.studocu.com
Anatomy questions (13) Anatomy Practice Questions (Acids, Bases, and Ph Buffer Questions A weak acid and it’s conjugate base in equilibrium: the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Definition a buffer solution is one which. in this section, we will see how to perform calculations to predict the ph at any point in a titration. Ph Buffer Questions.
From www.slideserve.com
PPT Friday, April 5 PowerPoint Presentation, free download ID1910051 Ph Buffer Questions A weak acid and it’s conjugate base in equilibrium: ph buffers the basic idea: practice calculating the ph of a buffer with practice problems and explanations. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. A buffer solution is a mixture of a weak acid and. Ph Buffer Questions.
From www.homeworklib.com
C. pH of Buffer Solutions (continued) Measured pH Calculated pH (from Ph Buffer Questions A weak acid and it’s conjugate base in equilibrium: A buffer solution is a mixture of a weak acid and its conjugate base. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. It resists any change in the ph upon the addition of acidic or basic. practice. Ph Buffer Questions.
From www.chegg.com
Solved ?You Need To Make A Buffer Whose PH Is 7.0, And Yo... Ph Buffer Questions this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. It resists any change in the ph upon the addition of acidic or basic. A weak acid and it’s conjugate base in equilibrium: this page describes simple acidic and alkaline buffer solutions and explains how they work. . Ph Buffer Questions.
From www.scribd.com
pH and Buffers Acid Buffer Solution Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. A weak acid and it’s conjugate base in equilibrium: Definition a buffer solution is one which. this is a summary practice problem. Ph Buffer Questions.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Ph Buffer Questions in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. practice calculating the ph of a buffer with practice problems and explanations. Definition a buffer solution is one which. ph buffers the basic idea: this page describes simple acidic and. Ph Buffer Questions.
From www.chegg.com
Solved A student must make a buffer solution with a pH of Ph Buffer Questions What is a buffer solution? A weak acid and it’s conjugate base in equilibrium: Definition a buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. practice calculating the ph of a buffer with practice problems and explanations. ph buffers the basic idea: the ability of a. Ph Buffer Questions.
From www.youtube.com
17.2 Calculating pH of Buffer Solutions YouTube Ph Buffer Questions Definition a buffer solution is one which. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. this page describes simple acidic and alkaline buffer solutions and explains how they work. the ability of a buffer solution to resist large changes. Ph Buffer Questions.
From mmerevise.co.uk
pH Curves Questions and Revision MME Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. It resists any change in the ph upon the addition of acidic or basic. Definition a buffer solution is one which. A buffer solution is a mixture of a weak acid and its conjugate base. What is. Ph Buffer Questions.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. this is a summary practice problem set on buffer. Ph Buffer Questions.
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube Ph Buffer Questions A weak acid and it’s conjugate base in equilibrium: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. Definition a buffer solution is one which.. Ph Buffer Questions.
From www.chegg.com
Solved You need to make a buffer whose pH is 9.0, and you Ph Buffer Questions practice calculating the ph of a buffer with practice problems and explanations. A weak acid and it’s conjugate base in equilibrium: It resists any change in the ph upon the addition of acidic or basic. Definition a buffer solution is one which. the ability of a buffer solution to resist large changes in ph has a great many. Ph Buffer Questions.
From www.chegg.com
Solved Desk Date Hydrolysis of Salts and pH of Buffer Ph Buffer Questions What is a buffer solution? ph buffers the basic idea: It resists any change in the ph upon the addition of acidic or basic. Definition a buffer solution is one which. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a. this page describes simple acidic and. Ph Buffer Questions.
From study.com
Quiz & Worksheet How to Calculate the pH of a Buffered Solution Ph Buffer Questions ph buffers the basic idea: What is a buffer solution? the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. practice calculating the ph of a buffer with practice problems and explanations. this page describes simple acidic and alkaline buffer solutions and explains how. Ph Buffer Questions.
From cewzpojc.blob.core.windows.net
Buffer Questions at Sandra Kell blog Ph Buffer Questions It resists any change in the ph upon the addition of acidic or basic. practice calculating the ph of a buffer with practice problems and explanations. Definition a buffer solution is one which. A weak acid and it’s conjugate base in equilibrium: this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating. Ph Buffer Questions.
From www.chegg.com
Solved C.3 Observe the trends in pH for both buffer Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. practice calculating the ph of a buffer with practice problems and explanations. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Ph Buffer Questions.
From www.chegg.com
Solved 13. a. A buffer solution has pH=5 and pKa=5.3. What Ph Buffer Questions the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps the most. this page describes simple acidic and alkaline buffer solutions and explains how they work. in this section, we will see how to perform calculations to predict the ph at any point in a titration of. Ph Buffer Questions.