What Is The Meaning Of Clock Reaction . A clock reaction can be used to measure the rate of reaction in certain circumstances. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. In a typical reaction the initial rate (at t=0) doesn’t.
from studylib.net
clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. A clock reaction can be used to measure the rate of reaction in certain circumstances. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well.
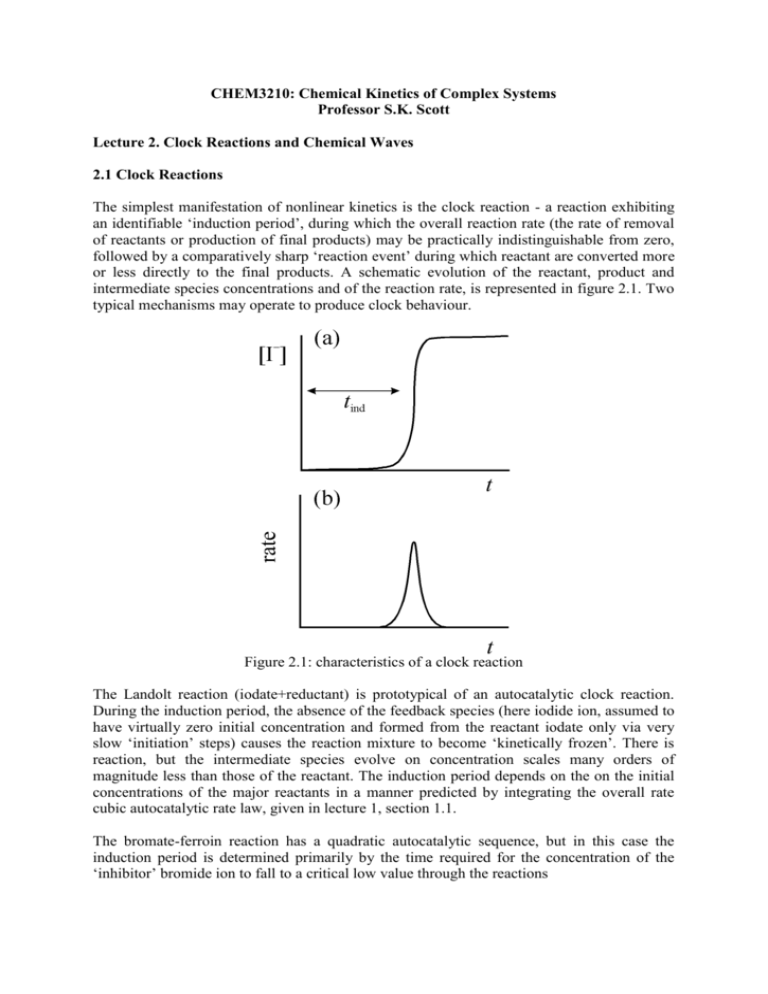
Lecture 2. Clock Reactions and Chemical Waves
What Is The Meaning Of Clock Reaction clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. A clock reaction can be used to measure the rate of reaction in certain circumstances. In a typical reaction the initial rate (at t=0) doesn’t. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to.
From www.kingmariot.ae
Clock reaction King Mariot Medical Equipment What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. clear distinction should be made between the several different causes that may lead to an induction. What Is The Meaning Of Clock Reaction.
From melscience.com
Iodine clock reaction MEL Chemistry What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. clear distinction should be made between the several different. What Is The Meaning Of Clock Reaction.
From www.wikihow.com
How to Perform the Iodine Clock Reaction 11 Steps (with Pictures) What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several. What Is The Meaning Of Clock Reaction.
From sciencenotes.org
Halloween Clock Reaction Old Nassau Reaction What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. In a typical reaction the initial rate (at t=0) doesn’t. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because. What Is The Meaning Of Clock Reaction.
From chemistry.stackexchange.com
reaction mechanism What reagents change the time of a chemical clock What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. this reaction rate is. What Is The Meaning Of Clock Reaction.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. A clock reaction can be used to measure the rate of reaction in certain circumstances. In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction is a simplified version of the traditional initial rates. What Is The Meaning Of Clock Reaction.
From www.chegg.com
Solved Rates of Chemical Reactions A Clock Reaction What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. In a typical reaction the. What Is The Meaning Of Clock Reaction.
From www.chegg.com
Study on the “Clock Reaction” 1. Objectives What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. this reaction rate is a measure of. What Is The Meaning Of Clock Reaction.
From www.savemyexams.co.uk
Iodine Clock Reaction (8.1.5) AQA A Level Chemistry Revision Notes What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clock. What Is The Meaning Of Clock Reaction.
From www.youtube.com
Chemical Clock Reaction YouTube What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because. What Is The Meaning Of Clock Reaction.
From symbolsage.com
Clock Symbolism What Does it Mean? Symbol Sage What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be. What Is The Meaning Of Clock Reaction.
From www.chemistrystudent.com
Clock Reactions and Iodine Clock Reaction (ALevel) ChemistryStudent What Is The Meaning Of Clock Reaction a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. A clock reaction can be used to measure the rate of reaction in certain circumstances. In a typical reaction the. What Is The Meaning Of Clock Reaction.
From caen-sccm-cdp01.engin.umich.edu
🌈 of the persulfate iodide clock reaction. Experiment 4 What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. . What Is The Meaning Of Clock Reaction.
From www.youtube.com
of a Clock Reaction Sample Data YouTube What Is The Meaning Of Clock Reaction clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. this reaction rate is a measure. What Is The Meaning Of Clock Reaction.
From www.scribd.com
Eh110a31 Lab2 a Clock Reaction Reaction Rate Chemical Reactions What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because they show a sharp dramatic colour change after a period. What Is The Meaning Of Clock Reaction.
From www.scribd.com
iodine clock reaction Reaction Rate Chemical Reactions What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. . What Is The Meaning Of Clock Reaction.
From www.studypool.com
SOLUTION CHEM 163 of Iodine Clock Reaction Lab Report Studypool What Is The Meaning Of Clock Reaction clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. In a typical reaction the initial rate (at t=0) doesn’t. clock reactions are so called because they show a. What Is The Meaning Of Clock Reaction.
From crunchchemistry.co.uk
What is a clock reaction? Crunch Chemistry What Is The Meaning Of Clock Reaction a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. A clock reaction can be used to measure the rate of reaction in certain circumstances. this reaction rate is. What Is The Meaning Of Clock Reaction.
From www.chegg.com
Solved Rates of Chemical Reactions A Clock Reaction What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. In a typical reaction the initial rate (at t=0) doesn’t. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to.. What Is The Meaning Of Clock Reaction.
From studylib.net
Lecture 2. Clock Reactions and Chemical Waves What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. A clock reaction can be used to measure the rate of reaction in certain circumstances. this. What Is The Meaning Of Clock Reaction.
From www.scribd.com
Iodine Clock Reaction What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. a clock reaction is a simplified version of the traditional initial rates method to determine orders. What Is The Meaning Of Clock Reaction.
From www.researchgate.net
(PDF) What is and what isn’t a clock reaction? What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. A clock reaction can be used to. What Is The Meaning Of Clock Reaction.
From www.youtube.com
of the Iodine Clock Reaction Intro & Theory YouTube What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. a clock. What Is The Meaning Of Clock Reaction.
From www.youtube.com
The Clock Reaction YouTube What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well.. What Is The Meaning Of Clock Reaction.
From studylib.net
Rates of Chemical Reactions I A Clock Reaction What Is The Meaning Of Clock Reaction clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. A clock reaction can be used to measure the rate of reaction in certain circumstances. In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction (or chemical clock) is a type of chemical reaction in. What Is The Meaning Of Clock Reaction.
From oneclass.com
OneClass Il. A Clock Reaction 1. A student studied the clock reaction What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. a. What Is The Meaning Of Clock Reaction.
From www.cuemath.com
Analog Clock with Minutes Basics, Definitions, Examples Cuemath What Is The Meaning Of Clock Reaction A clock reaction can be used to measure the rate of reaction in certain circumstances. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. this reaction rate is. What Is The Meaning Of Clock Reaction.
From www.researchgate.net
(PDF) Clock Reactions What Is The Meaning Of Clock Reaction clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. this reaction rate is a measure of how much iodine was produced in the time it took. What Is The Meaning Of Clock Reaction.
From www.jlcatj.gob.mx
Clock Reaction Outlet Wholesale, Save 44 jlcatj.gob.mx What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. clear distinction should be made between the several different causes that may lead to an induction. What Is The Meaning Of Clock Reaction.
From rosagoodue.blogspot.com
The Endpoint of the Clock Reaction for This Experiment Is the What Is The Meaning Of Clock Reaction clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several different. What Is The Meaning Of Clock Reaction.
From studylib.net
topic 16 iodine clock reaction What Is The Meaning Of Clock Reaction a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. A clock reaction can be used to measure the rate of reaction in certain circumstances. In a. What Is The Meaning Of Clock Reaction.
From www.coursehero.com
of a clock reaction reaction rates 1, 2, 3,.... Course Hero What Is The Meaning Of Clock Reaction a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. In a typical reaction the initial rate (at t=0) doesn’t. this reaction rate is a measure of how much iodine was produced in the time it took for the reaction to turn blue (i.e., time taken to. clear distinction. What Is The Meaning Of Clock Reaction.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free What Is The Meaning Of Clock Reaction clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. a clock reaction is a simplified version of the traditional initial rates method to determine orders. What Is The Meaning Of Clock Reaction.
From steamdaily.com
10 Amazing Chemical Reactions What Is The Meaning Of Clock Reaction In a typical reaction the initial rate (at t=0) doesn’t. a clock reaction (or chemical clock) is a type of chemical reaction in which the product appears suddenly with a well. a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several. What Is The Meaning Of Clock Reaction.
From www.slideserve.com
PPT Chemical Lab The formaldehyde clock reaction PowerPoint What Is The Meaning Of Clock Reaction a clock reaction is a simplified version of the traditional initial rates method to determine orders of reaction. clear distinction should be made between the several different causes that may lead to an induction period in a kinetic. clock reactions are so called because they show a sharp dramatic colour change after a period of time has. What Is The Meaning Of Clock Reaction.