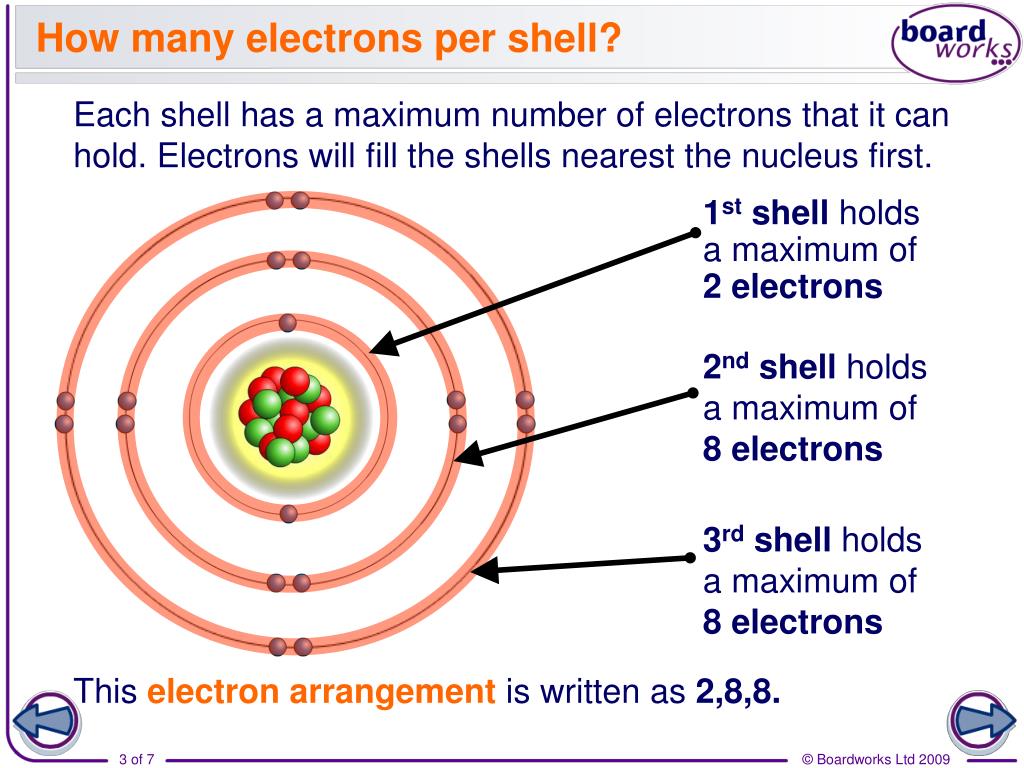
Electron Shell Rules . Each allowed electron orbit is. As we discussed previously, this does. Electron shells have one or. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; An electron shell is the outside part of an atom around the atomic nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Bohr model of the atom. In each term of an. How to write electron configurations. The energy levels for the electrons in an atom are often referred to as electron shells. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron orbitals and orbital shapes. It is a group of atomic orbitals with the same value of the principal quantum number n. Topics covered in other articles.
from www.slideserve.com
Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In each term of an. It is a group of atomic orbitals with the same value of the principal quantum number n. Electron orbitals and orbital shapes. How to write electron configurations. The energy levels for the electrons in an atom are often referred to as electron shells. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Bohr model of the atom. Topics covered in other articles. As we discussed previously, this does.
PPT How are electrons arranged? PowerPoint Presentation, free
Electron Shell Rules An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In each term of an. An electron shell is the outside part of an atom around the atomic nucleus. Each allowed electron orbit is. How to write electron configurations. Electron orbitals and orbital shapes. As we discussed previously, this does. Topics covered in other articles. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. It is a group of atomic orbitals with the same value of the principal quantum number n. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Bohr model of the atom. The energy levels for the electrons in an atom are often referred to as electron shells. Electron shells have one or.
From mmerevise.co.uk
Atoms Questions and Revision MME Electron Shell Rules An electron shell is the outside part of an atom around the atomic nucleus. How to write electron configurations. It is a group of atomic orbitals with the same value of the principal quantum number n. Topics covered in other articles. Electron shells have one or. An electron shell is the set of allowed states that share the same principal. Electron Shell Rules.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Electron Shell Rules It is a group of atomic orbitals with the same value of the principal quantum number n. Electron orbitals and orbital shapes. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. In each term of an. In this tutorial, you will learn about electron shells, the different subshells,. Electron Shell Rules.
From gzscienceclassonline.weebly.com
1. Electron Configuration Electron Shell Rules The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; As we discussed previously, this does. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Bohr model of the atom. How to write electron configurations. An. Electron Shell Rules.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Electron Shell Rules Each allowed electron orbit is. In each term of an. Bohr model of the atom. Electron orbitals and orbital shapes. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may. Electron Shell Rules.
From www.slideserve.com
PPT VALENCE ELECTRONS & BONDING PowerPoint Presentation, free Electron Shell Rules It is a group of atomic orbitals with the same value of the principal quantum number n. Each allowed electron orbit is. How to write electron configurations. As we discussed previously, this does. The energy levels for the electrons in an atom are often referred to as electron shells. Electron orbitals and orbital shapes. In each term of an. Electron. Electron Shell Rules.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Electron Shell Rules Bohr model of the atom. The energy levels for the electrons in an atom are often referred to as electron shells. Electron orbitals and orbital shapes. As we discussed previously, this does. An electron shell is the outside part of an atom around the atomic nucleus. In this tutorial, you will learn about electron shells, the different subshells, and the. Electron Shell Rules.
From www.scienceabc.com
Noble Metal Definition, List, Properties, And Examples Electron Shell Rules The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Topics covered in other articles. The energy levels for the electrons in an atom are often. Electron Shell Rules.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Electron Shell Rules An electron shell is the outside part of an atom around the atomic nucleus. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each allowed electron orbit is. How to write electron configurations. It is. Electron Shell Rules.
From diagramdataspongious.z21.web.core.windows.net
Electron Shell Diagram For Carbon Electron Shell Rules An electron shell is the outside part of an atom around the atomic nucleus. Topics covered in other articles. How to write electron configurations. Each allowed electron orbit is. Bohr model of the atom. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. As we discussed previously, this. Electron Shell Rules.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Electron Shell Rules As we discussed previously, this does. Bohr model of the atom. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electron orbitals and orbital shapes. In each term of an. How to write electron configurations.. Electron Shell Rules.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Electron Shell Rules An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. An electron shell is the outside part of an atom around the atomic nucleus. Bohr model of the atom. As we discussed previously, this does. Electron shells have one or. Each allowed electron orbit is. In each term of. Electron Shell Rules.
From www.youtube.com
Distribution of electron in K L M N Shell II Bohr & bury rule Il Electron Shell Rules As we discussed previously, this does. It is a group of atomic orbitals with the same value of the principal quantum number n. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron orbitals and orbital shapes. An electron shell is the outside part of an atom around. Electron Shell Rules.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png Electron Shell Rules The energy levels for the electrons in an atom are often referred to as electron shells. Each allowed electron orbit is. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; An electron shell is the set of allowed states that share the same principal quantum number,. Electron Shell Rules.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry Electron Shell Rules As we discussed previously, this does. An electron shell is the outside part of an atom around the atomic nucleus. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; An electron shell is the set of allowed states that share the same principal quantum number, n,. Electron Shell Rules.
From www.thoughtco.com
Electron Configuration Chart Electron Shell Rules It is a group of atomic orbitals with the same value of the principal quantum number n. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. The general. Electron Shell Rules.
From www.tes.com
Chemistryteacher001's Shop Teaching Resources TES Electron Shell Rules Electron orbitals and orbital shapes. Each allowed electron orbit is. It is a group of atomic orbitals with the same value of the principal quantum number n. Bohr model of the atom. As we discussed previously, this does. In each term of an. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons. Electron Shell Rules.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID6667703 Electron Shell Rules The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Bohr model of the atom. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. As we discussed previously, this does. Topics covered in other articles.. Electron Shell Rules.
From slidetodoc.com
electron shells a Atomic number number of Electrons Electron Shell Rules The energy levels for the electrons in an atom are often referred to as electron shells. Topics covered in other articles. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands. Electron Shell Rules.
From proper-cooking.info
Periodic Table Of Elements With Electron Shells Electron Shell Rules How to write electron configurations. It is a group of atomic orbitals with the same value of the principal quantum number n. Topics covered in other articles. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Bohr model of the atom. An electron shell is the outside part of. Electron Shell Rules.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Electron Shell Rules Bohr model of the atom. In each term of an. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; An electron shell is the outside part of an atom around the atomic nucleus. As we discussed previously, this does. It is a group of atomic orbitals. Electron Shell Rules.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Electron Shell Rules The energy levels for the electrons in an atom are often referred to as electron shells. An electron shell is the outside part of an atom around the atomic nucleus. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electron shells have one or. An electron shell is the set of allowed states that share the. Electron Shell Rules.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Electron Shell Rules Topics covered in other articles. An electron shell is the outside part of an atom around the atomic nucleus. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Each allowed electron orbit is. As we discussed previously, this does. Electron shell, regions surrounding the atomic nucleus. Electron Shell Rules.
From www.slideserve.com
PPT Electron’s in Atoms PowerPoint Presentation, free download ID Electron Shell Rules In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Topics covered in other articles. In each term of an. Electron orbitals and orbital shapes. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. An electron shell is. Electron Shell Rules.
From www.britannica.com
Electron shell Definition & Facts Britannica Electron Shell Rules Bohr model of the atom. How to write electron configurations. The energy levels for the electrons in an atom are often referred to as electron shells. In each term of an. Topics covered in other articles. An electron shell is the outside part of an atom around the atomic nucleus. The general rule stating the number of electrons present in. Electron Shell Rules.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Electron Shell Rules Electron orbitals and orbital shapes. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. As we discussed previously, this does. How to write electron configurations. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; Each. Electron Shell Rules.
From www.animalia-life.club
Electron Shell Diagram Electron Shell Rules An electron shell is the outside part of an atom around the atomic nucleus. Topics covered in other articles. As we discussed previously, this does. In each term of an. Each allowed electron orbit is. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. Electron shell, regions surrounding. Electron Shell Rules.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Electron Shell Rules Bohr model of the atom. How to write electron configurations. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. An electron shell is the outside part of an atom around the atomic nucleus. Each allowed electron orbit is. Electron orbitals and orbital shapes. In this tutorial, you will. Electron Shell Rules.
From www.slideserve.com
PPT Structure of Atom PowerPoint Presentation, free download ID6503501 Electron Shell Rules Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Bohr model of the atom. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. In. Electron Shell Rules.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Electron Shell Rules How to write electron configurations. The energy levels for the electrons in an atom are often referred to as electron shells. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. It is a group of atomic orbitals with the same value of the principal quantum number n. As we. Electron Shell Rules.
From slidetodoc.com
Why are electrons important Electrons are responsible for Electron Shell Rules Topics covered in other articles. Each allowed electron orbit is. An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number n. In each term of an. The energy levels for the electrons in an atom are often referred to as. Electron Shell Rules.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Electron Shell Rules The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for the number of shells; In each term of an. Electron shells have one or. How to write electron configurations. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Topics covered. Electron Shell Rules.
From www.pinterest.com
Valence Electrons• The electrons in the outer most electron shell are Electron Shell Rules In each term of an. Electron shells have one or. An electron shell is the outside part of an atom around the atomic nucleus. An electron shell is the set of allowed states that share the same principal quantum number, n, that electrons may occupy. It is a group of atomic orbitals with the same value of the principal quantum. Electron Shell Rules.
From www.youtube.com
mr i explains Electron Shells (GCSE Chemistry) YouTube Electron Shell Rules In each term of an. How to write electron configurations. As we discussed previously, this does. It is a group of atomic orbitals with the same value of the principal quantum number n. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. An electron shell is the set of. Electron Shell Rules.
From byjus.com
What are the rules we follow while writing electron shell structure how Electron Shell Rules Topics covered in other articles. In this tutorial, you will learn about electron shells, the different subshells, and the orbitals where electrons can be found. Each allowed electron orbit is. The energy levels for the electrons in an atom are often referred to as electron shells. Electron shells have one or. It is a group of atomic orbitals with the. Electron Shell Rules.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Electron Shell Rules Bohr model of the atom. How to write electron configurations. Electron orbitals and orbital shapes. In each term of an. The energy levels for the electrons in an atom are often referred to as electron shells. Topics covered in other articles. The general rule stating the number of electrons present in a shell is 2n 2, where ‘n’ stands for. Electron Shell Rules.