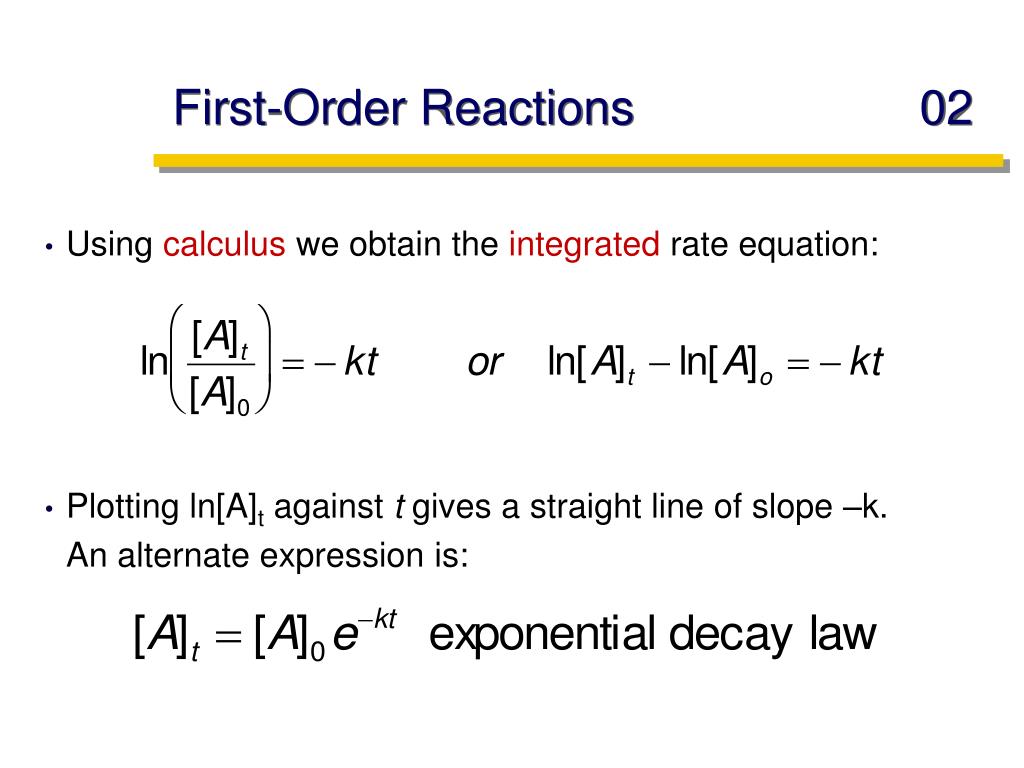
Rate Constant First Order Reaction Chemistry . The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is the reaction order. If n = 0, the. In which [a], [b], and.
from mavink.com
In other words, if the concentration is doubled, the reaction rate is. The integrated rate law for a first. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. In which [a], [b], and. If n = 0, the.
First Order Reaction Units
Rate Constant First Order Reaction Chemistry If n = 0, the. Where k is the rate constant and n is the reaction order. If n = 0, the. In other words, if the concentration is doubled, the reaction rate is. In which [a], [b], and. Our objective is to determine the reaction order by calculating the n from a set of experiments. The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form:
From kunduz.com
[ANSWERED] If the rate constant for a first order re... Physical Rate Constant First Order Reaction Chemistry The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. In which. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
The rate constant for a first order reaction six times when the Rate Constant First Order Reaction Chemistry In which [a], [b], and. In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: The integrated rate law for a first. Where k. Rate Constant First Order Reaction Chemistry.
From www.brainkart.com
Rate equation for first order reactions Rate Constant First Order Reaction Chemistry In other words, if the concentration is doubled, the reaction rate is. Where k is the rate constant and n is the reaction order. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: If n. Rate Constant First Order Reaction Chemistry.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. If n = 0, the. The integrated rate law for a first. Where k is the rate constant and n is the reaction order. In general, a rate law (or differential. Rate Constant First Order Reaction Chemistry.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant First Order Reaction Chemistry In which [a], [b], and. The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction. Rate Constant First Order Reaction Chemistry.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second Rate Constant First Order Reaction Chemistry If n = 0, the. In which [a], [b], and. Where k is the rate constant and n is the reaction order. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if. Rate Constant First Order Reaction Chemistry.
From ar.inspiredpencil.com
First Order Reaction Rate Rate Constant First Order Reaction Chemistry In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. In which [a], [b], and. Our objective is to determine the reaction order by calculating the n from a set of experiments. The integrated rate law for a first. If n. Rate Constant First Order Reaction Chemistry.
From 9to5science.com
[Solved] Formula for rate constant for the first order 9to5Science Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. If n = 0, the. In which [a], [b], and. The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is. Rate Constant First Order Reaction Chemistry.
From socratic.org
What is the halflife of a firstorder reaction with a rate constant of Rate Constant First Order Reaction Chemistry The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is the reaction order. In which [a], [b], and. In other. Rate Constant First Order Reaction Chemistry.
From chemguru.sg
Rate Equation and Order of Reaction Rate Constant First Order Reaction Chemistry In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. In which [a], [b], and. The integrated rate law for a first. Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant First Order Reaction Chemistry The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. Where k. Rate Constant First Order Reaction Chemistry.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps Rate Constant First Order Reaction Chemistry In which [a], [b], and. In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is. Rate Constant First Order Reaction Chemistry.
From ar.inspiredpencil.com
First Order Reaction Rate Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. If n = 0, the. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In which [a], [b], and. The integrated rate law. Rate Constant First Order Reaction Chemistry.
From galldemax10.netlify.app
First Order Integrated Rate Law Equation Gallery Dedemax Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is the reaction order. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: If n = 0, the. In other words, if the concentration is doubled, the. Rate Constant First Order Reaction Chemistry.
From byjus.com
What is the unit of rate constant for first order reaction Rate Constant First Order Reaction Chemistry In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction order by calculating the n from a set of experiments. If n = 0, the. The integrated rate law for a first. In which [a], [b], and. In general, a rate law (or differential rate law, as it is sometimes called). Rate Constant First Order Reaction Chemistry.
From www.brainkart.com
Rate equation for first order reactions Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is the reaction order. If n = 0, the. The integrated rate law for a first. In which [a], [b], and. In other words, if the concentration is doubled, the reaction rate is. In general,. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Rate Constant First Order Reaction Chemistry In other words, if the concentration is doubled, the reaction rate is. In which [a], [b], and. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a. Rate Constant First Order Reaction Chemistry.
From www.meritnation.com
The rate constant of a first order reaction increases from 2 X 102 to Rate Constant First Order Reaction Chemistry Where k is the rate constant and n is the reaction order. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. In general, a rate law (or differential rate law, as it is sometimes called). Rate Constant First Order Reaction Chemistry.
From www.tessshebaylo.com
Derive Integrated Rate Equation For A First Order Gas Phase Reaction Rate Constant First Order Reaction Chemistry In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. In which [a], [b], and. The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a. Rate Constant First Order Reaction Chemistry.
From www.researchgate.net
How to calculate rate constant for first order reaction? Rate Constant First Order Reaction Chemistry The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is the reaction order. In which [a], [b], and. In other words, if the concentration is doubled, the reaction rate is. In general,. Rate Constant First Order Reaction Chemistry.
From study.com
First Order Reaction & Rate Law Definition, Equation & Examples Rate Constant First Order Reaction Chemistry Where k is the rate constant and n is the reaction order. Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: The integrated rate law for a first. If n = 0, the. In which. Rate Constant First Order Reaction Chemistry.
From askfilo.com
The rate constant for a first order reaction is 20 min−1. The time requir.. Rate Constant First Order Reaction Chemistry If n = 0, the. Where k is the rate constant and n is the reaction order. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. In which [a], [b], and. The integrated rate law for a first. Our objective. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
first order reaction rate calculation example YouTube Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. The integrated rate law for a first. In which [a], [b], and. If n = 0, the. In other words, if the concentration is doubled, the reaction rate is. Where k is the rate constant and n is the reaction order. In general,. Rate Constant First Order Reaction Chemistry.
From askfilo.com
CHEMICAL 1. The specific rate constant of first order reaction i.. Rate Constant First Order Reaction Chemistry The integrated rate law for a first. Where k is the rate constant and n is the reaction order. If n = 0, the. In which [a], [b], and. Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. In general,. Rate Constant First Order Reaction Chemistry.
From mavink.com
First Order Reaction Units Rate Constant First Order Reaction Chemistry The integrated rate law for a first. In which [a], [b], and. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a. Rate Constant First Order Reaction Chemistry.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 Rate Constant First Order Reaction Chemistry In which [a], [b], and. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In other words, if the concentration is doubled, the reaction rate is. Where k is the rate constant and n is the reaction order. Our objective is to determine the reaction order by calculating the n from a. Rate Constant First Order Reaction Chemistry.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: In which [a], [b], and. Where k is the rate constant and n is the reaction order. The integrated rate law for a first. If n. Rate Constant First Order Reaction Chemistry.
From study.com
Identifying HalfLife Given the Rate Constant Chemistry Rate Constant First Order Reaction Chemistry In which [a], [b], and. If n = 0, the. The integrated rate law for a first. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Our objective is to determine the reaction order by calculating the n from a set of experiments. Where k is the rate constant and n is. Rate Constant First Order Reaction Chemistry.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction Rate Constant First Order Reaction Chemistry If n = 0, the. In other words, if the concentration is doubled, the reaction rate is. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Our objective is to determine the reaction order by calculating the n from a set of experiments. In which [a], [b], and. Where k is the. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant First Order Reaction Chemistry If n = 0, the. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Where k is the rate constant and n is the reaction order. In other words, if the concentration is doubled, the reaction rate is. Our objective is to determine the reaction order by calculating the n from a. Rate Constant First Order Reaction Chemistry.
From www.brainkart.com
Rate equation for first order reactions Rate Constant First Order Reaction Chemistry In which [a], [b], and. Where k is the rate constant and n is the reaction order. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. The integrated rate law for a first. In other words, if the concentration is doubled, the reaction rate is. In general,. Rate Constant First Order Reaction Chemistry.
From www.coursehero.com
[Solved] A certain firstorder reaction has a rate constant of Rate Constant First Order Reaction Chemistry In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Our objective is to determine the reaction order by calculating the n from a set of experiments. If n = 0, the. In which [a], [b], and. In other words, if the concentration is doubled, the reaction rate is. The integrated rate law. Rate Constant First Order Reaction Chemistry.
From www.youtube.com
Rate Constant for First Order Reaction Problem Chemical Rate Constant First Order Reaction Chemistry Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. Where k is the rate constant and n is the reaction order. The integrated rate law for a first. In general, a rate law (or differential rate law, as it is. Rate Constant First Order Reaction Chemistry.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant First Order Reaction Chemistry In which [a], [b], and. Where k is the rate constant and n is the reaction order. The integrated rate law for a first. If n = 0, the. Our objective is to determine the reaction order by calculating the n from a set of experiments. In other words, if the concentration is doubled, the reaction rate is. In general,. Rate Constant First Order Reaction Chemistry.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Rate Constant First Order Reaction Chemistry Where k is the rate constant and n is the reaction order. In general, a rate law (or differential rate law, as it is sometimes called) takes this form: Our objective is to determine the reaction order by calculating the n from a set of experiments. In which [a], [b], and. The integrated rate law for a first. In other. Rate Constant First Order Reaction Chemistry.