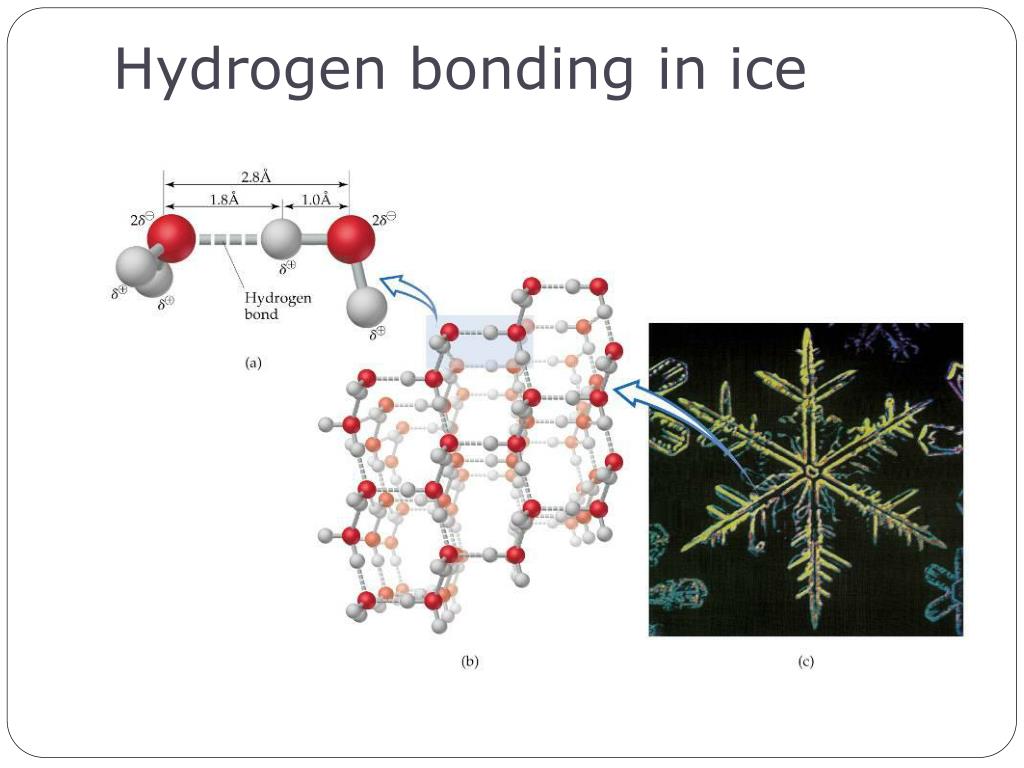
Why Ice Float Hydrogen Bond . This produces a crystal lattice commonly known as ice. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. When water is warm, the. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). The hexagonal channels become partially filled, and the volume. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ice is less dense than liquid water and so it floats.
from mungfali.com
As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). This produces a crystal lattice commonly known as ice. When water is warm, the. The hexagonal channels become partially filled, and the volume. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable.
Ice Hydrogen Bonding
Why Ice Float Hydrogen Bond The hexagonal channels become partially filled, and the volume. This produces a crystal lattice commonly known as ice. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. When water is warm, the. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. The hexagonal channels become partially filled, and the volume. Ice is less dense than liquid water and so it floats.
From slideplayer.com
Biology Concepts & Applications ppt download Why Ice Float Hydrogen Bond Ice is less dense than liquid water and so it floats. When water is warm, the. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). This produces a crystal lattice commonly. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. The hexagonal channels. Why Ice Float Hydrogen Bond.
From mungfali.com
Ice Hydrogen Bonding Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Water molecules form hydrogen bonds when. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Solids differ Hardness Melting point Flexibility Conductivity Why Ice Float Hydrogen Bond And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water is warm, the. The molecules in ice are held further. Why Ice Float Hydrogen Bond.
From ar.inspiredpencil.com
Hydrogen Bond In Ice Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. Ice is less dense than liquid water and so it floats. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water. Why Ice Float Hydrogen Bond.
From slideplayer.com
Essential Chemistry for Biology ppt download Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water is. Why Ice Float Hydrogen Bond.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Ice Float Hydrogen Bond The hexagonal channels become partially filled, and the volume. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. When water is warm, the. And at temperatures low enough to turn off the disruptive effects. Why Ice Float Hydrogen Bond.
From slideplayer.com
Essential Chemistry for Biology ppt download Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. When water is warm, the. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another. Why Ice Float Hydrogen Bond.
From ar.inspiredpencil.com
Hydrogen Bonding In Ice Why Ice Float Hydrogen Bond And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. When water is warm, the. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). The hexagonal channels become partially filled, and. Why Ice Float Hydrogen Bond.
From mungfali.com
Ice Hydrogen Bonding Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. When water is warm, the. Water molecules form hydrogen bonds when an oxygen. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. This. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water is warm, the. Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. When ice melts, some of the. Why Ice Float Hydrogen Bond.
From mungfali.com
Ice Hydrogen Bonding Why Ice Float Hydrogen Bond The hexagonal channels become partially filled, and the volume. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Intermolecular Force PowerPoint Presentation, free download ID Why Ice Float Hydrogen Bond When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. And at temperatures low. Why Ice Float Hydrogen Bond.
From www.youtube.com
MDCAT55 Structure of ice Hydrogen bonds in ice Ice floats in Why Ice Float Hydrogen Bond The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ice is less dense than liquid water and so it floats. This produces a crystal lattice commonly known as ice. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). As the. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. When water is warm, the. The hexagonal channels become partially filled, and the volume. When ice melts, some of the hydrogen. Why Ice Float Hydrogen Bond.
From slideplayer.com
4 From Chemistry to Energy to Life Part A ppt download Why Ice Float Hydrogen Bond When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free Why Ice Float Hydrogen Bond When water is warm, the. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. The hexagonal channels become partially filled, and the volume. The molecules in ice are held further. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The molecules in ice are held further apart by the hydrogen bonds between the. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Ice is less dense than liquid water and so it floats. When water is warm, the. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom. Why Ice Float Hydrogen Bond.
From mungfali.com
Ice Hydrogen Bonding Why Ice Float Hydrogen Bond When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Ice is less dense than liquid water and so it floats. And at. Why Ice Float Hydrogen Bond.
From www.youtube.com
Why Does Ice Float?!? Intermolecular Forces Explained Hydrogen Bonding Why Ice Float Hydrogen Bond Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). This produces a crystal lattice commonly known as ice. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Ice is less dense than liquid water and so it floats. As the water. Why Ice Float Hydrogen Bond.
From mungfali.com
Ice Hydrogen Bonding Why Ice Float Hydrogen Bond Ice is less dense than liquid water and so it floats. The hexagonal channels become partially filled, and the volume. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. When water is. Why Ice Float Hydrogen Bond.
From slideplayer.com
CHAPTER 9 Water and Solutions 9.1 Solutes, Solvents, and Water. ppt Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. This produces a crystal lattice commonly known as ice. Ice is less dense than liquid water and so it floats. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form. Why Ice Float Hydrogen Bond.
From www.pinterest.com
Scientific Explanation of Why Ice Floats Science for kids, Hydrogen Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. This produces a crystal lattice commonly known as ice. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. And at temperatures low enough to turn off the disruptive effects of thermal motions, water. Why Ice Float Hydrogen Bond.
From www.chemistrynotmystery.com
Hydrogen Bond Chemistry!!! Not Mystery Why Ice Float Hydrogen Bond The hexagonal channels become partially filled, and the volume. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When ice melts, some of the hydrogen bonds are broken and the rigid. Why Ice Float Hydrogen Bond.
From slideplayer.com
CHEMICAL CONTEXT OF LIFE ppt download Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water is. Why Ice Float Hydrogen Bond.
From animalia-life.club
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond When water is warm, the. Ice is less dense than liquid water and so it floats. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. Water molecules form hydrogen bonds when an. Why Ice Float Hydrogen Bond.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on Why Ice Float Hydrogen Bond As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Intermolecular Force PowerPoint Presentation, free download ID Why Ice Float Hydrogen Bond The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). When water is warm, the.. Why Ice Float Hydrogen Bond.
From www.agefotostock.com
Why Does Ice Float on Water Infographic Diagram showing different in Why Ice Float Hydrogen Bond The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water is warm, the. As the water cools to below 4°c, the hydrogen bonds adjust to hold the negatively charged oxygen atoms apart. This produces a crystal lattice commonly known as ice. Water molecules form hydrogen bonds when an oxygen atom. Why Ice Float Hydrogen Bond.
From www.slideserve.com
PPT Chapter 2 Lecture Notes—Essential Chemistry for Biology Biol 100 Why Ice Float Hydrogen Bond When water is warm, the. Ice is less dense than liquid water and so it floats. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. This produces a crystal lattice commonly known as ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms.. Why Ice Float Hydrogen Bond.
From ar.inspiredpencil.com
Hydrogen Bonds Ice Why Ice Float Hydrogen Bond And at temperatures low enough to turn off the disruptive effects of thermal motions, water freezes into ice in which the hydrogen bonds form a rigid and stable. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. This produces a crystal lattice commonly known as ice. Water molecules form hydrogen bonds when. Why Ice Float Hydrogen Bond.
From www.sliderbase.com
Hydrogen Bonding & Water Why Ice Float Hydrogen Bond This produces a crystal lattice commonly known as ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. When water is warm, the. When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. And at temperatures low enough to turn off the disruptive effects. Why Ice Float Hydrogen Bond.