Bromine Trifluoride Intermolecular Forces . chemspider record containing structure,. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. the chemical formula for this interhalogen compound is brf 3. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Brf3 is a polar molecule due to the. Br 2 + 3f 2 → 2brf 3. The table gives the melting. intermolecular forces affect melting temperatures, boiling temperatures and solubility. The reaction is represented by the following equation: Bromine is a liquid at room temperature.
from animalia-life.club
Brf3 is a polar molecule due to the. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. The reaction is represented by the following equation: thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Br 2 + 3f 2 → 2brf 3. Bromine is a liquid at room temperature. the chemical formula for this interhalogen compound is brf 3. intermolecular forces affect melting temperatures, boiling temperatures and solubility. The table gives the melting.
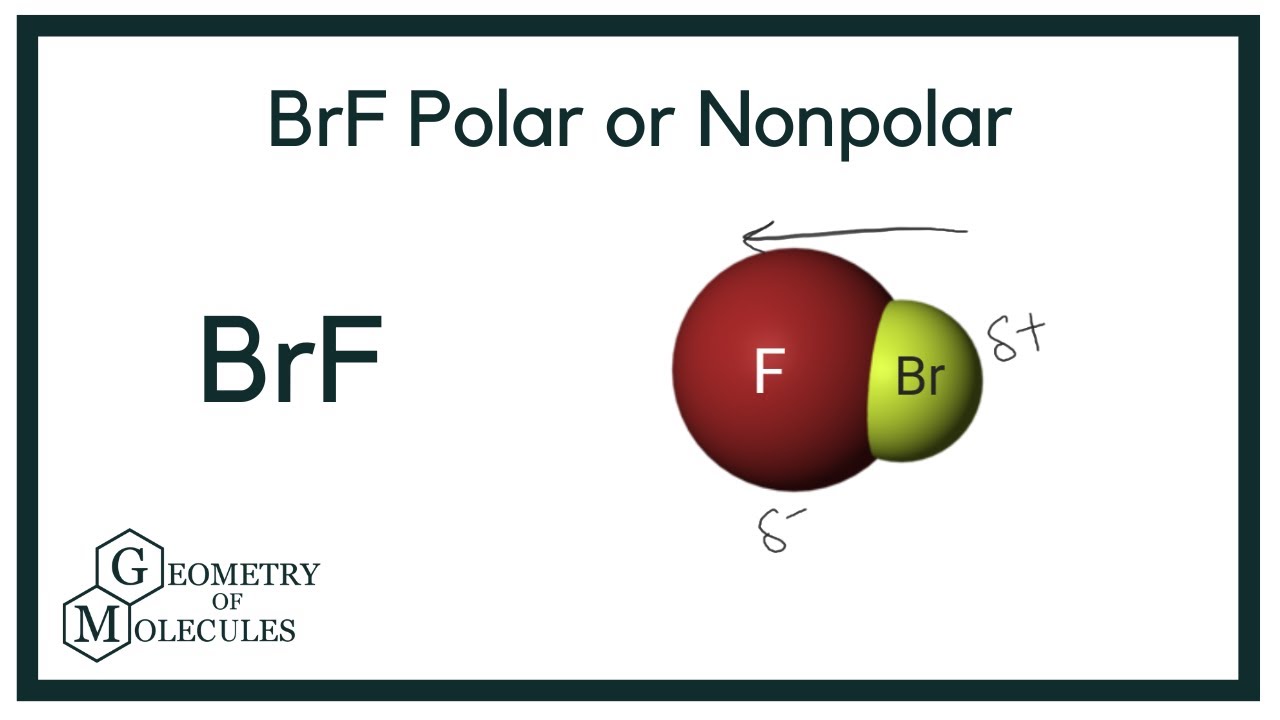
Brf3 Polar Or Nonpolar
Bromine Trifluoride Intermolecular Forces In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Bromine is a liquid at room temperature. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. The reaction is represented by the following equation: the chemical formula for this interhalogen compound is brf 3. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Br 2 + 3f 2 → 2brf 3. Brf3 is a polar molecule due to the. intermolecular forces affect melting temperatures, boiling temperatures and solubility. The table gives the melting. chemspider record containing structure,.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Intermolecular Forces the chemical formula for this interhalogen compound is brf 3. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. chemspider record containing structure,. Br 2 + 3f 2 → 2brf 3. The table gives the melting. The reaction is represented by the following equation:. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromine Trifluoride Intermolecular Forces chemspider record containing structure,. the chemical formula for this interhalogen compound is brf 3. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Br 2 + 3f 2 → 2brf 3. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. The table gives the melting.. Bromine Trifluoride Intermolecular Forces.
From www.numerade.com
SOLVED Draw the best Lewis structure and give the polarity Bromine Trifluoride Intermolecular Forces It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Br 2 + 3f 2 → 2brf 3. Bromine is a liquid at room temperature. intermolecular forces affect melting temperatures, boiling temperatures and solubility. In the year 1906,. Bromine Trifluoride Intermolecular Forces.
From www.numerade.com
SOLVED What volume of bromine is produced when 94.4 liters of bromine Bromine Trifluoride Intermolecular Forces intermolecular forces affect melting temperatures, boiling temperatures and solubility. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. chemspider record containing structure,. Bromine is a liquid at room temperature. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Br. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Determine the Bond Type (Electronegativities) ppt download Bromine Trifluoride Intermolecular Forces The table gives the melting. The reaction is represented by the following equation: Brf3 is a polar molecule due to the. Bromine is a liquid at room temperature. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. thus, diatomic bromine does not have any intermolecular. Bromine Trifluoride Intermolecular Forces.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Intermolecular Forces thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. the chemical formula for this interhalogen compound is brf 3. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. The table gives the melting. intermolecular forces affect melting temperatures, boiling temperatures and solubility. chemspider record. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromine Trifluoride Intermolecular Forces It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. The reaction is represented by the following equation: The table gives the melting. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Brf3 is a polar molecule due to. Bromine Trifluoride Intermolecular Forces.
From www.numerade.com
SOLVED Which answer best describes the strongest intermolecular force Bromine Trifluoride Intermolecular Forces The reaction is represented by the following equation: Br 2 + 3f 2 → 2brf 3. intermolecular forces affect melting temperatures, boiling temperatures and solubility. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. the chemical formula for this interhalogen compound is brf 3. In the year 1906, paul lebeau. Bromine Trifluoride Intermolecular Forces.
From slidetodoc.com
Polarity and Intermolecular Forces Types of bonds Ionic Bromine Trifluoride Intermolecular Forces It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. intermolecular forces affect melting temperatures, boiling temperatures and solubility. Br 2 + 3f 2 → 2brf 3. Brf3 is a polar molecule due to the. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Intermolecular Forces Notes ppt download Bromine Trifluoride Intermolecular Forces chemspider record containing structure,. The reaction is represented by the following equation: It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Bromine is a liquid at room temperature. Brf3 is a polar molecule due to the. the chemical formula for this interhalogen compound is brf 3. thus, diatomic bromine. Bromine Trifluoride Intermolecular Forces.
From in.pinterest.com
Bromine trifluoride has the chemical formula BrF3 and interhalogen Bromine Trifluoride Intermolecular Forces chemspider record containing structure,. The reaction is represented by the following equation: intermolecular forces affect melting temperatures, boiling temperatures and solubility. Brf3 is a polar molecule due to the. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. It is unlikely to be a. Bromine Trifluoride Intermolecular Forces.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube Bromine Trifluoride Intermolecular Forces Brf3 is a polar molecule due to the. Br 2 + 3f 2 → 2brf 3. The table gives the melting. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. intermolecular forces affect melting temperatures, boiling temperatures and solubility. Bromine is a liquid at room. Bromine Trifluoride Intermolecular Forces.
From www.chemtube3d.com
BrF3 Bromine trifluoride Bromine Trifluoride Intermolecular Forces thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. The reaction is represented by the following equation: Brf3 is a polar molecule due to the. Br 2 + 3f 2 → 2brf 3. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. the chemical formula for. Bromine Trifluoride Intermolecular Forces.
From www.youtube.com
Lewis Structure of BrF3 (bromine trifluoride) YouTube Bromine Trifluoride Intermolecular Forces Brf3 is a polar molecule due to the. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Br 2 + 3f 2 → 2brf 3. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. It is unlikely to be a solid. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromine Trifluoride Intermolecular Forces Bromine is a liquid at room temperature. The reaction is represented by the following equation: Brf3 is a polar molecule due to the. chemspider record containing structure,. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. intermolecular forces affect melting temperatures, boiling temperatures and. Bromine Trifluoride Intermolecular Forces.
From www.youtube.com
Is BrF3 (Bromine trifluoride) Ionic or Covalent/Molecular? YouTube Bromine Trifluoride Intermolecular Forces thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. chemspider record containing structure,. Bromine is a liquid at room temperature. intermolecular forces affect melting temperatures, boiling temperatures and solubility. The reaction is represented by the following equation: It is unlikely to be a solid at room temperature unless the dispersion forces are strong. Bromine Trifluoride Intermolecular Forces.
From animalia-life.club
Brf3 Polar Or Nonpolar Bromine Trifluoride Intermolecular Forces the chemical formula for this interhalogen compound is brf 3. Bromine is a liquid at room temperature. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. The reaction is represented by the following equation: chemspider record containing structure,. The table gives the melting. intermolecular forces affect melting temperatures, boiling. Bromine Trifluoride Intermolecular Forces.
From www.youtube.com
BrF3 Bromine Trifluoride Shape Hybridisation VSEPR Problem Bromine Trifluoride Intermolecular Forces chemspider record containing structure,. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Br 2 + 3f 2 → 2brf 3. The reaction is represented by the following equation: the chemical formula for this interhalogen compound is brf 3. Brf3 is a polar molecule due to the. thus, diatomic. Bromine Trifluoride Intermolecular Forces.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Intermolecular Forces The reaction is represented by the following equation: The table gives the melting. Bromine is a liquid at room temperature. the chemical formula for this interhalogen compound is brf 3. Br 2 + 3f 2 → 2brf 3. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature. Bromine Trifluoride Intermolecular Forces.
From www.numerade.com
SOLVED Determine which intermolecular forces act between the molecules Bromine Trifluoride Intermolecular Forces Br 2 + 3f 2 → 2brf 3. The reaction is represented by the following equation: The table gives the melting. chemspider record containing structure,. intermolecular forces affect melting temperatures, boiling temperatures and solubility. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In the year 1906, paul lebeau synthesized this compound for. Bromine Trifluoride Intermolecular Forces.
From whatsinsight.org
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight Bromine Trifluoride Intermolecular Forces In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. chemspider record containing structure,. The reaction is represented by the following equation: thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. intermolecular forces affect melting temperatures, boiling temperatures and solubility.. Bromine Trifluoride Intermolecular Forces.
From whatsinsight.org
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight Bromine Trifluoride Intermolecular Forces Brf3 is a polar molecule due to the. the chemical formula for this interhalogen compound is brf 3. The table gives the melting. Br 2 + 3f 2 → 2brf 3. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In the year 1906, paul lebeau synthesized this compound for the first time by. Bromine Trifluoride Intermolecular Forces.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Intermolecular Forces The table gives the melting. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. Brf3 is a polar molecule due to the. Br 2 + 3f 2 → 2brf 3. chemspider record containing structure,. intermolecular forces affect melting temperatures, boiling temperatures and solubility. It is unlikely to be a solid at room temperature. Bromine Trifluoride Intermolecular Forces.
From www.bartleby.com
Answered Decide which intermolecular forces act… bartleby Bromine Trifluoride Intermolecular Forces Brf3 is a polar molecule due to the. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Br 2 + 3f 2 → 2brf 3. the chemical formula for this interhalogen compound is brf 3. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In the. Bromine Trifluoride Intermolecular Forces.
From www.ch.imperial.ac.uk
VSEPR Theory A closer look at bromine trifluoride, BrF3. Henry Rzepa Bromine Trifluoride Intermolecular Forces The table gives the melting. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Brf3 is a polar molecule due to the. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. thus, diatomic bromine does not have. Bromine Trifluoride Intermolecular Forces.
From hanenhuusholli.blogspot.com
Lewis Dot Diagram For Bromine Hanenhuusholli Bromine Trifluoride Intermolecular Forces It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. the chemical formula for this interhalogen compound is brf 3. Bromine is a liquid at room temperature. Br 2 + 3f 2 → 2brf 3. The reaction is represented by the following equation: thus, diatomic bromine does not have any intermolecular. Bromine Trifluoride Intermolecular Forces.
From www.doubtnut.com
Draw structure and name the shape of bromine trifluoride Bromine Trifluoride Intermolecular Forces intermolecular forces affect melting temperatures, boiling temperatures and solubility. The table gives the melting. The reaction is represented by the following equation: Brf3 is a polar molecule due to the. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. chemspider record containing structure,. Br 2 + 3f 2 → 2brf. Bromine Trifluoride Intermolecular Forces.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Trifluoride Intermolecular Forces It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. the chemical formula for this interhalogen compound is brf 3. chemspider record containing structure,. The table gives the melting. Bromine is a liquid at room temperature. Brf3 is a polar molecule due to the. thus, diatomic bromine does not have. Bromine Trifluoride Intermolecular Forces.
From imgbin.com
Bromine Pentafluoride Bromine Trifluoride Lewis Structure Bromate PNG Bromine Trifluoride Intermolecular Forces The reaction is represented by the following equation: It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. chemspider record containing structure,. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Bromine is a liquid at room temperature.. Bromine Trifluoride Intermolecular Forces.
From www.pinterest.com
BrF3 Molecular Geometry, Bond Angles(Bromine Trifluoride) Molecular Bromine Trifluoride Intermolecular Forces The reaction is represented by the following equation: The table gives the melting. Bromine is a liquid at room temperature. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. the chemical formula for this interhalogen compound is brf 3. Brf3 is a polar molecule due to the. Br 2 + 3f 2 → 2brf. Bromine Trifluoride Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Bromine Trifluoride Intermolecular Forces The table gives the melting. the chemical formula for this interhalogen compound is brf 3. thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Bromine is a liquid at room temperature.. Bromine Trifluoride Intermolecular Forces.
From www.chegg.com
Solved Name bromine trifluoride ∇ Bromine Trifluoride Intermolecular Forces The reaction is represented by the following equation: chemspider record containing structure,. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. Br 2 + 3f 2 → 2brf 3. Bromine is a liquid at room temperature. thus, diatomic bromine does not have any intermolecular. Bromine Trifluoride Intermolecular Forces.
From www.numerade.com
SOLVED Decide which intermolecular forces act between the molecules of Bromine Trifluoride Intermolecular Forces The table gives the melting. The reaction is represented by the following equation: thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. the chemical formula for this interhalogen compound is brf 3. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of. Bromine Trifluoride Intermolecular Forces.
From quizlet.com
What is the hybridization of the central atom in the bromine Quizlet Bromine Trifluoride Intermolecular Forces thus, diatomic bromine does not have any intermolecular forces other than dispersion forces. the chemical formula for this interhalogen compound is brf 3. intermolecular forces affect melting temperatures, boiling temperatures and solubility. It is unlikely to be a solid at room temperature unless the dispersion forces are strong enough. Br 2 + 3f 2 → 2brf 3.. Bromine Trifluoride Intermolecular Forces.
From www.youtube.com
Intermolecular Forces for NF3 YouTube Bromine Trifluoride Intermolecular Forces The table gives the melting. In the year 1906, paul lebeau synthesized this compound for the first time by reacting bromine with fluorine at a temperature of 20°c. The reaction is represented by the following equation: intermolecular forces affect melting temperatures, boiling temperatures and solubility. Brf3 is a polar molecule due to the. It is unlikely to be a. Bromine Trifluoride Intermolecular Forces.