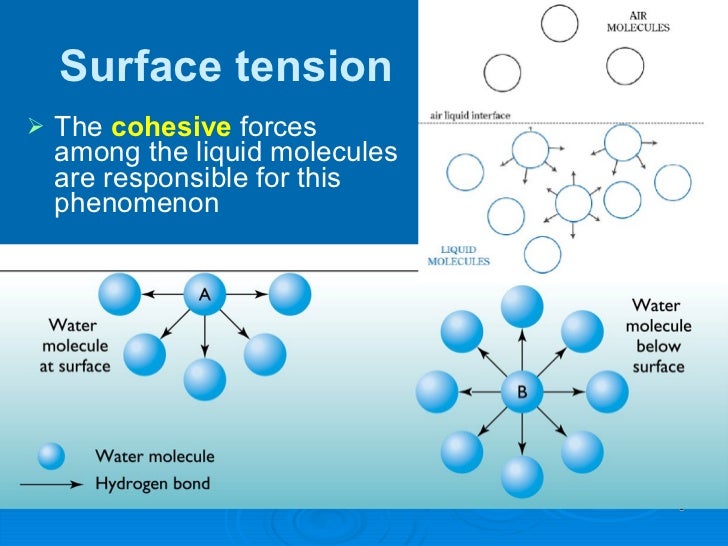
What Is The Relative Surface Tension Of Water . Understand cohesive and adhesive forces. • surface tension is a property of a liquid that allows them to resist external forces. Equivalently, it can be stated as surface energy in ergs. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols ,. By the end of this section, you will be able to: surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. It combines the concepts of cohesion and adhesion. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from www.slideshare.net
water has high surface tension, which can be explained by its polarity and hydrogen bonding. Understand cohesive and adhesive forces. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols ,. By the end of this section, you will be able to: Equivalently, it can be stated as surface energy in ergs. • surface tension is a property of a liquid that allows them to resist external forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water consists of one oxygen atom flanked by. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Measuring the Surface Tension of Water by Light Diffraction on Capill…
What Is The Relative Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Understand cohesive and adhesive forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs. In comparison, organic liquids, such as benzene and alcohols ,. It combines the concepts of cohesion and adhesion. By the end of this section, you will be able to: water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water consists of one oxygen atom flanked by. • surface tension is a property of a liquid that allows them to resist external forces.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is The Relative Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. Water consists of one oxygen atom flanked by. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). By the end of this section, you will be able to: surface tension is the energy, or work, required to increase the surface area. What Is The Relative Surface Tension Of Water.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is The Relative Surface Tension Of Water Equivalently, it can be stated as surface energy in ergs. It combines the concepts of cohesion and adhesion. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water consists of one oxygen atom flanked by. • surface tension is a property of a liquid that allows them. What Is The Relative Surface Tension Of Water.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download What Is The Relative Surface Tension Of Water Equivalently, it can be stated as surface energy in ergs. • surface tension is a property of a liquid that allows them to resist external forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Understand cohesive and adhesive forces. water has a surface tension of. What Is The Relative Surface Tension Of Water.
From mungfali.com
Water Surface Tension Bubble What Is The Relative Surface Tension Of Water • surface tension is a property of a liquid that allows them to resist external forces. In comparison, organic liquids, such as benzene and alcohols ,. Equivalently, it can be stated as surface energy in ergs. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). By the end of this section,. What Is The Relative Surface Tension Of Water.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Is The Relative Surface Tension Of Water Water consists of one oxygen atom flanked by. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c. What Is The Relative Surface Tension Of Water.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. • surface tension is a property of a liquid that allows them to resist external forces. Equivalently, it can be stated as surface energy in ergs. water has high surface tension, which can be explained by its polarity and hydrogen bonding. By the end of this section, you will be able to: Water. What Is The Relative Surface Tension Of Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Is The Relative Surface Tension Of Water It combines the concepts of cohesion and adhesion. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. • surface tension is a property of a liquid that allows them to. What Is The Relative Surface Tension Of Water.
From giozhnupc.blob.core.windows.net
How Is Surface Tension Of Water Important To Life at James Ebert blog What Is The Relative Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Equivalently, it can be stated as surface energy in ergs. Water consists of one oxygen atom flanked by. water has high surface tension, which can be explained by its polarity and hydrogen. What Is The Relative Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is The Relative Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It combines the concepts of cohesion and adhesion. Water consists of one oxygen atom flanked by. Equivalently, it can be stated as. What Is The Relative Surface Tension Of Water.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is The Relative Surface Tension Of Water It combines the concepts of cohesion and adhesion. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols ,. Water consists of one oxygen atom flanked by. Equivalently,. What Is The Relative Surface Tension Of Water.
From www.youtube.com
Surface Tension of Water Explained YouTube What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. In comparison, organic liquids, such as benzene and alcohols ,. By the end of this section, you will be able to: water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid. What Is The Relative Surface Tension Of Water.
From www.researchgate.net
Calculated surface tension of wateroil with respect to temperature What Is The Relative Surface Tension Of Water Water consists of one oxygen atom flanked by. By the end of this section, you will be able to: surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs. surface tension is typically measured in dynes/cm, the force. What Is The Relative Surface Tension Of Water.
From byjus.com
Explain the surface tension phenomenon with examples. What Is The Relative Surface Tension Of Water • surface tension is a property of a liquid that allows them to resist external forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen. What Is The Relative Surface Tension Of Water.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Is The Relative Surface Tension Of Water Equivalently, it can be stated as surface energy in ergs. It combines the concepts of cohesion and adhesion. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). • surface tension is a property of a. What Is The Relative Surface Tension Of Water.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Is The Relative Surface Tension Of Water It combines the concepts of cohesion and adhesion. By the end of this section, you will be able to: Water consists of one oxygen atom flanked by. Understand cohesive and adhesive forces. • surface tension is a property of a liquid that allows them to resist external forces. Equivalently, it can be stated as surface energy in ergs. . What Is The Relative Surface Tension Of Water.
From mavink.com
Water Surface Tension Chart What Is The Relative Surface Tension Of Water Water consists of one oxygen atom flanked by. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has high surface tension, which can be explained by its polarity and hydrogen bonding. It combines the concepts of cohesion and adhesion. By the end of this section, you. What Is The Relative Surface Tension Of Water.
From mydigitalkemistry.com
What is Surface Tension Explain with Example Best Online Free What Is The Relative Surface Tension Of Water By the end of this section, you will be able to: It combines the concepts of cohesion and adhesion. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Equivalently, it can be. What Is The Relative Surface Tension Of Water.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? What Is The Relative Surface Tension Of Water It combines the concepts of cohesion and adhesion. Water consists of one oxygen atom flanked by. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. Understand cohesive and adhesive forces. By the end of this section,. What Is The Relative Surface Tension Of Water.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical What Is The Relative Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Understand cohesive. What Is The Relative Surface Tension Of Water.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. By the end of this section, you will be able to: water has high surface tension, which can be explained by its polarity and hydrogen bonding. Equivalently, it can be stated as surface energy in ergs. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water. What Is The Relative Surface Tension Of Water.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Is The Relative Surface Tension Of Water By the end of this section, you will be able to: Understand cohesive and adhesive forces. Equivalently, it can be stated as surface energy in ergs. It combines the concepts of cohesion and adhesion. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene and alcohols ,.. What Is The Relative Surface Tension Of Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is The Relative Surface Tension Of Water Equivalently, it can be stated as surface energy in ergs. Understand cohesive and adhesive forces. It combines the concepts of cohesion and adhesion. In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). By the end of this section, you will be able. What Is The Relative Surface Tension Of Water.
From www.researchgate.net
2 Schematic representation of surface tension of a hemispherical water What Is The Relative Surface Tension Of Water water has high surface tension, which can be explained by its polarity and hydrogen bonding. By the end of this section, you will be able to: Understand cohesive and adhesive forces. Equivalently, it can be stated as surface energy in ergs. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It. What Is The Relative Surface Tension Of Water.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water consists of one oxygen atom flanked by. surface tension is typically measured in dynes/cm, the force. What Is The Relative Surface Tension Of Water.
From www.researchgate.net
Surface tension (a) and relative surface tension (b) defined as σ = σ What Is The Relative Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by. surface tension is the. What Is The Relative Surface Tension Of Water.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Is The Relative Surface Tension Of Water It combines the concepts of cohesion and adhesion. Understand cohesive and adhesive forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to. What Is The Relative Surface Tension Of Water.
From www.researchgate.net
Surface tension of water. Download Scientific Diagram What Is The Relative Surface Tension Of Water surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Understand cohesive and adhesive forces. It combines the concepts of cohesion and adhesion. Equivalently, it can be stated as surface energy in ergs. surface tension is typically measured in dynes/cm, the force in dynes required to break a. What Is The Relative Surface Tension Of Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Is The Relative Surface Tension Of Water water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. It combines the concepts of cohesion and adhesion. By the end of this section, you will be able to: Water consists of. What Is The Relative Surface Tension Of Water.
From fyofzwebh.blob.core.windows.net
Surface Tension Values Of Different Liquids at Lawrence Johnson blog What Is The Relative Surface Tension Of Water Water consists of one oxygen atom flanked by. Understand cohesive and adhesive forces. Equivalently, it can be stated as surface energy in ergs. In comparison, organic liquids, such as benzene and alcohols ,. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy,. What Is The Relative Surface Tension Of Water.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. By the end of this section, you will be able to: It combines the concepts of cohesion and adhesion. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. • surface tension is a property of a liquid that allows them to resist. What Is The Relative Surface Tension Of Water.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Is The Relative Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface. What Is The Relative Surface Tension Of Water.
From blog.biolinscientific.com
What is surface tension? What Is The Relative Surface Tension Of Water By the end of this section, you will be able to: Water consists of one oxygen atom flanked by. It combines the concepts of cohesion and adhesion. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid. What Is The Relative Surface Tension Of Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is The Relative Surface Tension Of Water In comparison, organic liquids, such as benzene and alcohols ,. By the end of this section, you will be able to: Understand cohesive and adhesive forces. It combines the concepts of cohesion and adhesion. • surface tension is a property of a liquid that allows them to resist external forces. water has high surface tension, which can be. What Is The Relative Surface Tension Of Water.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Is The Relative Surface Tension Of Water Equivalently, it can be stated as surface energy in ergs. Understand cohesive and adhesive forces. • surface tension is a property of a liquid that allows them to resist external forces. It combines the concepts of cohesion and adhesion. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1. What Is The Relative Surface Tension Of Water.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Is The Relative Surface Tension Of Water Understand cohesive and adhesive forces. water has high surface tension, which can be explained by its polarity and hydrogen bonding. • surface tension is a property of a liquid that allows them to resist external forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently,. What Is The Relative Surface Tension Of Water.