Calcium And Bromine Electronegativity . Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Values for electronegativity run from 0 to 4. The tendency of an atom to attract electrons to form a chemical bond. The electronegativity of an atom depends upon its atomic. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. The pauling scale is the most. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. The definition of electronegativity is: Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic.
from www.chegg.com
The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom depends upon its atomic. Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. The tendency of an atom to attract electrons to form a chemical bond. The pauling scale is the most. The definition of electronegativity is: Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself.
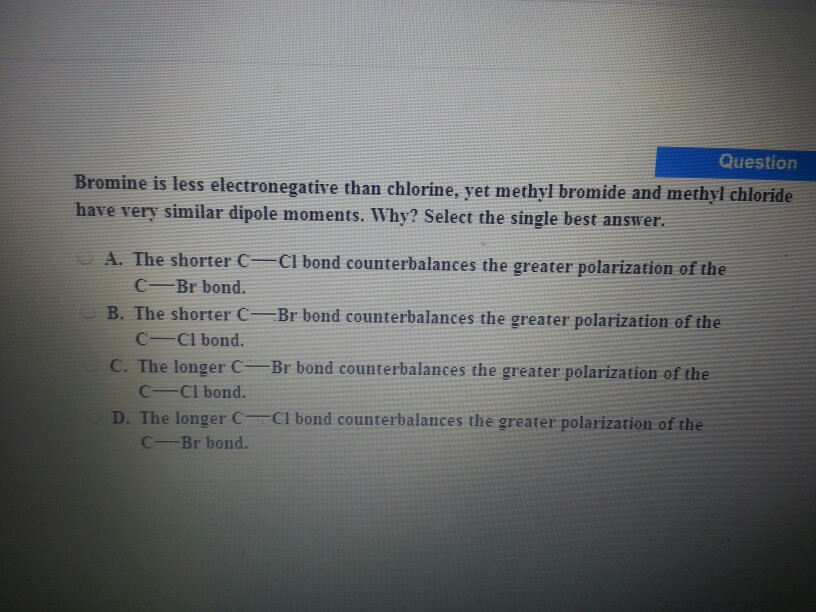
Solved Question Bromine is less electronegative than
Calcium And Bromine Electronegativity The electronegativity of an atom depends upon its atomic. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. The definition of electronegativity is: Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom depends upon its atomic. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. The pauling scale is the most. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Values for electronegativity run from 0 to 4. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. The tendency of an atom to attract electrons to form a chemical bond.
From www.numerade.com
SOLVED 1. Which orbital does not house core electrons for a bromine Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. The pauling scale is the most. Values for electronegativity run from 0 to 4. Compare calcium with bromine element of the. Calcium And Bromine Electronegativity.
From www.gauthmath.com
Solved 7) For each bond given, determine the bond type and rank in Calcium And Bromine Electronegativity The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. The definition. Calcium And Bromine Electronegativity.
From www.chegg.com
Solved 14. The electronegativity of bromine (Br) is 2.8. The Calcium And Bromine Electronegativity The definition of electronegativity is: Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Values for electronegativity. Calcium And Bromine Electronegativity.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Afbeelding 38153931 Calcium And Bromine Electronegativity The definition of electronegativity is: Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. The electronegativity of an atom depends upon its atomic. Compare calcium with bromine element of the. Calcium And Bromine Electronegativity.
From materialmediakay.z21.web.core.windows.net
Electronegativity Chart For Polarity Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The tendency of an atom to attract electrons to form a chemical bond. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls. Calcium And Bromine Electronegativity.
From www.numerade.com
SOLVED Part B Classify the following compounds as having covalent or Calcium And Bromine Electronegativity The tendency of an atom to attract electrons to form a chemical bond. Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a chemical property which describes how well an atom can attract. Calcium And Bromine Electronegativity.
From fyodefdzy.blob.core.windows.net
What Is The Order Of Electronegativity at Randy Tyler blog Calcium And Bromine Electronegativity Values for electronegativity run from 0 to 4. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a. Calcium And Bromine Electronegativity.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustratie Calcium And Bromine Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The tendency of an atom to attract electrons to form a chemical bond. The pauling scale is the most. Values for electronegativity run from 0 to 4. The definition of electronegativity is: Explore how electronegativity changes with atomic number in the periodic table. Calcium And Bromine Electronegativity.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Calcium And Bromine Electronegativity Values for electronegativity run from 0 to 4. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a chemical property which describes how well an atom can. Calcium And Bromine Electronegativity.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. The definition of electronegativity is: The tendency of an atom to attract electrons to form a chemical bond. The most used. Calcium And Bromine Electronegativity.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The most used definition of electronegativity is that an element's electronegativity is. Calcium And Bromine Electronegativity.
From www.chegg.com
Solved Question Bromine is less electronegative than Calcium And Bromine Electronegativity The pauling scale is the most. The tendency of an atom to attract electrons to form a chemical bond. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The most used definition of electronegativity is that. Calcium And Bromine Electronegativity.
From www.alamy.com
Bromine chemical element with first ionization energy, atomic mass and Calcium And Bromine Electronegativity Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Values for electronegativity run from 0 to 4. The electronegativity of an atom depends upon its atomic. The definition of electronegativity is: Electronegativity is. Calcium And Bromine Electronegativity.
From www.pinterest.com
Periodic Table Electronegativity Trend Ionization energy Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. The pauling scale is the most. The definition of electronegativity is: Values for electronegativity run from 0 to 4. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Explore how electronegativity changes with atomic number in the. Calcium And Bromine Electronegativity.
From brainly.com
which atom in each pair that has the greater electronegativity. a. Ca Calcium And Bromine Electronegativity Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. Values for electronegativity run from 0 to 4. The pauling scale is the most. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. 4 in this case, the electronegativity difference between calcium and bromine is. Calcium And Bromine Electronegativity.
From mavink.com
Element Electronegativity Chart Calcium And Bromine Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The definition of electronegativity is: The pauling scale is the most. The tendency of an atom to attract electrons to form a chemical bond. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Compare calcium with. Calcium And Bromine Electronegativity.
From www.numerade.com
SOLVED Which element meets all of the following conditions? has all Calcium And Bromine Electronegativity The electronegativity of an atom depends upon its atomic. Values for electronegativity run from 0 to 4. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The definition of. Calcium And Bromine Electronegativity.
From blog.naver.com
Electronegativity table, 전기음성도 표 네이버 블로그 Calcium And Bromine Electronegativity The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. The electronegativity of an atom depends upon its atomic.. Calcium And Bromine Electronegativity.
From www.pinterest.pt
periodic table with ionization energies Google Search Ionization Calcium And Bromine Electronegativity Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The electronegativity of an atom depends upon its atomic. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. The. Calcium And Bromine Electronegativity.
From greatjourneyto.com
Co powoduje polaryzację wiązań? Great Journey Calcium And Bromine Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. Compare calcium. Calcium And Bromine Electronegativity.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Calcium And Bromine Electronegativity The pauling scale is the most. The electronegativity of an atom depends upon its atomic. 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Electronegativity is a measure of the. Calcium And Bromine Electronegativity.
From bergmannchem.weebly.com
Documents Bergmann's chemistry Calcium And Bromine Electronegativity Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. The tendency of an atom to attract electrons to form a chemical bond. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. Values for electronegativity run from 0 to 4. The electronegativity of an atom depends. Calcium And Bromine Electronegativity.
From www.pinterest.com
Electronegativity Definition and Trend Calcium And Bromine Electronegativity The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The tendency of an atom to attract electrons to form a chemical bond. Compare calcium with bromine element of the periodic table on all their. Calcium And Bromine Electronegativity.
From www.questionai.com
the chart shows the electroniegativity values as Calcium And Bromine Electronegativity 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond 5. Compare calcium with bromine element of the periodic table on all their facts, electronic configuration, chemical, physical, atomic. The definition of electronegativity is: Electronegativity is a measure of the tendency of an atom to attract a. Calcium And Bromine Electronegativity.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Calcium And Bromine Electronegativity The electronegativity of an atom depends upon its atomic. The tendency of an atom to attract electrons to form a chemical bond. The most used definition of electronegativity is that an element's electronegativity is the power of an atom when. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is a measure. Calcium And Bromine Electronegativity.
From www.numerade.com
SOLVED Electronegativity and Electron Affinity Arrange the following Calcium And Bromine Electronegativity The definition of electronegativity is: Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. The pauling scale is the most. Values for electronegativity run from 0 to 4. The tendency of an atom to attract electrons to form a chemical bond. Explore how electronegativity changes with atomic number in the periodic table of elements. Calcium And Bromine Electronegativity.
From www.aiophotoz.com
Periodic Table Electronegativity Periodic Table Images and Photos finder Calcium And Bromine Electronegativity Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The pauling scale is the most. Electronegativity (pauling scale) → atomic radius decreases → ionization energy increases → electronegativity increases →. The electronegativity of an atom depends upon its atomic. The tendency of an atom to attract electrons to form a chemical bond.. Calcium And Bromine Electronegativity.
From printablecampusatokes.z21.web.core.windows.net
Electronegativity And Bond Polarity Chart Calcium And Bromine Electronegativity The pauling scale is the most. The electronegativity of an atom depends upon its atomic. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The definition of electronegativity is: 4 in this case, the electronegativity difference between calcium and bromine is 2.0, which falls within the range of a polar covalent bond. Calcium And Bromine Electronegativity.