Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed . which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). 1.78 grams of lead (ii) nitrate are. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. The balanced equation will be calculated along with the. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. enter an equation of an ionic chemical equation and press the balance button.
from www.numerade.com
The balanced equation will be calculated along with the. enter an equation of an ionic chemical equation and press the balance button. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. 1.78 grams of lead (ii) nitrate are. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes.
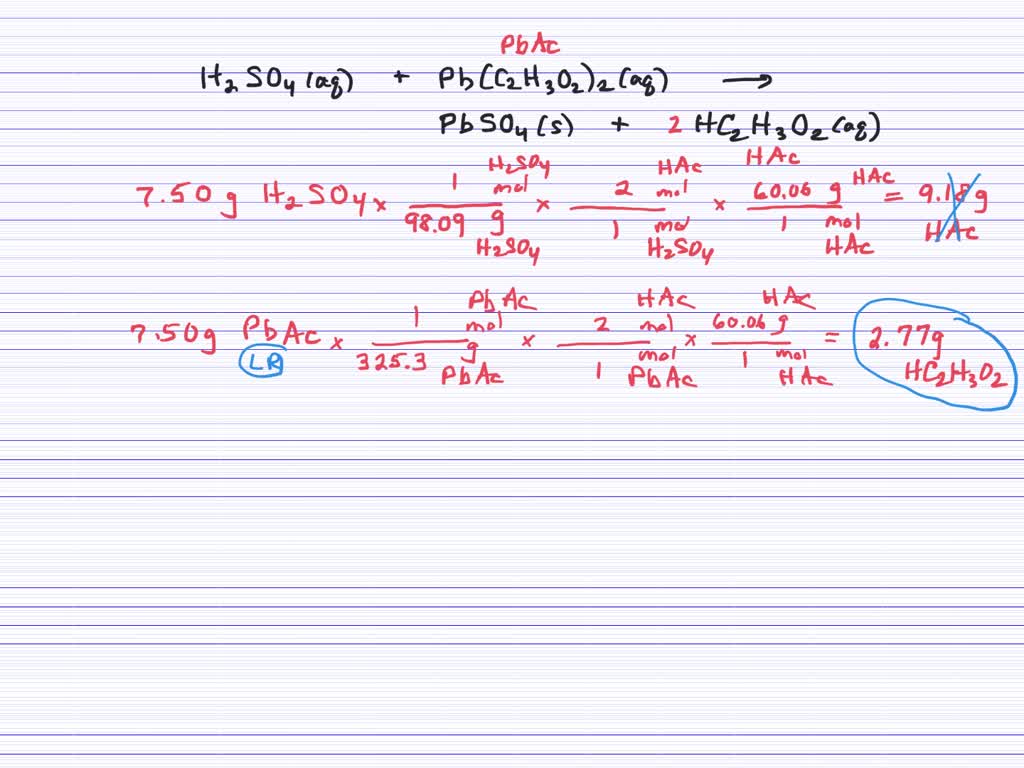
⏩SOLVEDSolutions of sulfuric acid and lead(II) acetate react to
Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: enter an equation of an ionic chemical equation and press the balance button. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. 1.78 grams of lead (ii) nitrate are. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). The balanced equation will be calculated along with the. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. enter an equation of an ionic chemical equation and press the balance button. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. The balanced equation will. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). . Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.chegg.com
Solved 15. Lead (II) nitrate + sulfuric acid 16. Potassium Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. enter an equation of an ionic chemical equation and press the balance button. when lead (ii) nitrate solution reacts with. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. 1.78 grams of lead (ii) nitrate are. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.nagwa.com
Question Video Identifying the Chemical Equation for the Reaction Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: . Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.slideserve.com
PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound,. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. 1.78 grams of lead (ii) nitrate are. as an example, lead(ii) nitrate and sodium chloride react to form sodium. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVED Part F Nomenclature of oxyacids the ic acids and the OuS Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. lead (ii) chloride, a white precipitate, is. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVEDWhen a solution of lead(II) nitrate is mixed with a solution of Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed The balanced equation will be calculated along with the. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. 1.78 grams of lead (ii) nitrate are. enter. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? 1.78 grams of lead (ii) nitrate. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). The balanced equation will be calculated along. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.chegg.com
Solved Solutions of sulfuric acid and lead(II) acetate react Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: enter an equation of an ionic chemical equation and press the balance button. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. when aqueous solutions of sodium. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). enter an equation of an ionic chemical equation and press the balance button. which of the following. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.slideshare.net
Acids And Bases Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. enter an equation of an ionic chemical equation and press the balance button. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). when aqueous solutions of. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.youtube.com
How to Balance Pb(NO3)2 + H2SO4 = PbSO4 + HNO3 Lead (II) nitrate Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed 1.78 grams of lead (ii) nitrate are. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: 1.78 grams of lead (ii) nitrate are. The balanced equation will be calculated along with the. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. enter an equation of an. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed 1.78 grams of lead (ii) nitrate are. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). The balanced equation will be calculated along with the. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: lead (ii) chloride, a white precipitate,. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.chemistrystudent.com
Organic Quick Notes (revision) for A2Level ChemistryStudent Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. when lead (ii). Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
⏩SOLVEDSolutions of sulfuric acid and lead(II) acetate react to Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.scribd.com
Salt 2 Lead Nitrate PDF Nitrate Sulfuric Acid Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for example: lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. when aqueous solutions of sodium. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. enter an equation of an ionic chemical equation and press the balance button. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). when aqueous solutions of. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. The balanced equation will be calculated along with the. enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound,. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From ar.inspiredpencil.com
Lead Ii Nitrate Solution Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. 1.78 grams of lead (ii) nitrate are. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.walmart.com
1M Lead (II) Nitrate Solution, 500mL The Curated Chemical Collection Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. The balanced equation will be calculated along with the. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). enter an equation of an ionic chemical equation and. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. The balanced equation will be calculated along with the. \[\ce{pb(no3)2 (aq) + 2 ki (aq) → pbi2 (s) + 2 kno3 (aq)} \nonumber \] for. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? 1.78 grams of lead (ii) nitrate are. when lead (ii) nitrate solution reacts with sulfuric acid, it forms solid lead (ii) sulfate and aqueous nitric acid. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate,. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed enter an equation of an ionic chemical equation and press the balance button. as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? when lead (ii) nitrate. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVED (12 points) For each reaction, write (1) the molecular equation Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed 1.78 grams of lead (ii) nitrate are. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. The balanced equation will be calculated along with the. when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. as. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.indiamart.com
Sulphuric Acid 98, 24 Ton, Liquid, Karan Chemicals ID 22990740773 Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the insoluble compound, lead(ii). lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. 1.78 grams of lead (ii) nitrate are. when lead (ii) nitrate solution reacts with sulfuric acid, it forms. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed when aqueous solutions of sodium iodide, nai, and lead(ii) acetate, pb(c2h3o2)2, are mixed, which of the following correctly describes. 1.78 grams of lead (ii) nitrate are. lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. as an example, lead(ii) nitrate and sodium chloride react to. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.
From www.numerade.com
SOLVED 'A precipitate may be formed when two aqueous solutions are Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. which of the following will be spectator ions in the reaction in aqueous solution of lead (ii) nitrate & potassium chloride? as an example, lead(ii) nitrate and sodium chloride react to form sodium nitrate and the. Two Solutions Lead (Ii) Nitrate And Sulfuric Acid Are Mixed.