Why Glasses Are Called Supercooled Liquids . here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. in the warm liquid several processes occur on different timescales. It is an amorphous solid—a state. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. liquids at temperatures below their melting points are called supercooled liquids. Can it be both and, if not, what. As described below, cooling a supercooled. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. Upon cooling the liquid below the melting point \.
from www.semanticscholar.org
As described below, cooling a supercooled. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Upon cooling the liquid below the melting point \. It is an amorphous solid—a state. liquids at temperatures below their melting points are called supercooled liquids. in the warm liquid several processes occur on different timescales. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. Can it be both and, if not, what. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid.
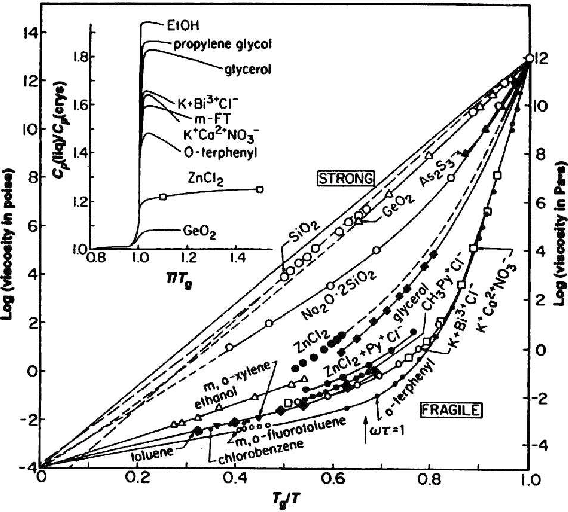
Figure 1 from Theory of structural glasses and supercooled liquids
Why Glasses Are Called Supercooled Liquids some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. It is an amorphous solid—a state. liquids at temperatures below their melting points are called supercooled liquids. As described below, cooling a supercooled. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Upon cooling the liquid below the melting point \. in the warm liquid several processes occur on different timescales. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. Can it be both and, if not, what. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered.
From www.teachmint.com
Supercooled Liquid Chemistry Notes Teachmint Why Glasses Are Called Supercooled Liquids It is an amorphous solid—a state. liquids at temperatures below their melting points are called supercooled liquids. Can it be both and, if not, what. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Upon cooling the liquid below the melting point \. glass, however, is. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Real Space Behavior of Supercooled Liquids, Glasses and Jamming systems Why Glasses Are Called Supercooled Liquids glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Upon cooling the liquid below the melting point \. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. It is an amorphous solid—a state. here we discuss current theoretical. Why Glasses Are Called Supercooled Liquids.
From www.engineeringnepal.com.np
GLASS SUPER COOL LIQUID Engineering Nepal Why Glasses Are Called Supercooled Liquids the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. in the warm liquid several processes occur on different timescales. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. Upon cooling the liquid below the melting. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Supercooled water demonstration explained YouTube Why Glasses Are Called Supercooled Liquids here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. As described below, cooling a supercooled. Upon cooling the liquid below the melting point \. Can it be both and, if not, what. glass is called a supercooled liquid because it retains the amorphous, disordered structure. Why Glasses Are Called Supercooled Liquids.
From www.thoughtco.com
Two Methods for Supercooling Water Why Glasses Are Called Supercooled Liquids Can it be both and, if not, what. As described below, cooling a supercooled. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. It is an amorphous solid—a state. in the warm liquid several processes occur on different timescales. here we discuss current theoretical knowledge of the manner in. Why Glasses Are Called Supercooled Liquids.
From www.semanticscholar.org
Figure 1 from Breaking Through the Glass Ceiling Recent Experimental Why Glasses Are Called Supercooled Liquids glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. liquids at temperatures. Why Glasses Are Called Supercooled Liquids.
From www.newscientist.com
Supercool experiment reveals water is actually two liquids in one New Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. in the warm liquid several processes occur on different timescales. liquids at temperatures below their melting points are called supercooled liquids. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. the opaque substance is made of metal alloys—made by. Why Glasses Are Called Supercooled Liquids.
From www.semanticscholar.org
Figure 1 from Theory of structural glasses and supercooled liquids Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Can it be both and, if not, what. liquids at temperatures below their melting. Why Glasses Are Called Supercooled Liquids.
From www.semanticscholar.org
Figure 1 from Molecular Dynamics Simulations of Supercooled Liquid Why Glasses Are Called Supercooled Liquids glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. It is an amorphous solid—a state. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. some liquids, because of complex molecular configuration or slow. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Why glass are called supercooled liquid YouTube Why Glasses Are Called Supercooled Liquids in the warm liquid several processes occur on different timescales. It is an amorphous solid—a state. Can it be both and, if not, what. As described below, cooling a supercooled. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. some liquids, because of complex. Why Glasses Are Called Supercooled Liquids.
From www.slideserve.com
PPT Supercooled liquids PowerPoint Presentation, free download ID Why Glasses Are Called Supercooled Liquids Upon cooling the liquid below the melting point \. As described below, cooling a supercooled. liquids at temperatures below their melting points are called supercooled liquids. in the warm liquid several processes occur on different timescales. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. Can. Why Glasses Are Called Supercooled Liquids.
From pubs.acs.org
Transformation of Stable Glasses into Supercooled Liquids Growth Why Glasses Are Called Supercooled Liquids glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. Can it be both and, if not, what. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Is Glass a Solid or a Liquid? 🤔 Why Glass is called Supercooled Why Glasses Are Called Supercooled Liquids some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. in the warm liquid several processes occur on different timescales. It is an amorphous solid—a state. liquids at temperatures below their melting points are called supercooled liquids. As described below, cooling a supercooled. Can it be both and, if not,. Why Glasses Are Called Supercooled Liquids.
From www.sciencerendezvous.ca
Science Rendezvous » Instant Ice Exploring Changes in States of Matter Why Glasses Are Called Supercooled Liquids the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. As described below, cooling a supercooled. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass is called a. Why Glasses Are Called Supercooled Liquids.
From www.semanticscholar.org
Figure 1 from Supercooled Liquids and Glasses Semantic Scholar Why Glasses Are Called Supercooled Liquids It is an amorphous solid—a state. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. Upon cooling the liquid below the melting point \. in the warm liquid several processes occur on different timescales. As described below, cooling a supercooled. liquids at temperatures below their melting points are. Why Glasses Are Called Supercooled Liquids.
From www.slideserve.com
PPT Supercooled liquids PowerPoint Presentation, free download ID Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. Can it be both and, if not, what. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. some liquids, because of complex molecular configuration or slow molecular. Why Glasses Are Called Supercooled Liquids.
From www.pnas.org
From particles to spins Eulerian formulation of supercooled liquids Why Glasses Are Called Supercooled Liquids some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. in. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Why is glass called a supercooled liquid? YouTube Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. liquids at temperatures below their melting points are called supercooled liquids. in the warm liquid several processes occur on different timescales. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. Upon cooling the liquid below the melting point \. Can it be. Why Glasses Are Called Supercooled Liquids.
From slideplayer.com
Potential Energy Landscape Description of Supercooled Liquids and Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. liquids at temperatures below their melting points are called supercooled liquids. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Can it be both and, if not, what. Upon cooling the liquid. Why Glasses Are Called Supercooled Liquids.
From twitter.com
ToughSF on Twitter "Vapordeposition of glasses enables the creation Why Glasses Are Called Supercooled Liquids liquids at temperatures below their melting points are called supercooled liquids. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. in the warm liquid several processes occur on different timescales. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass is called a supercooled liquid. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Supercooled liquid freezes instantly! YouTube Why Glasses Are Called Supercooled Liquids It is an amorphous solid—a state. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. Upon cooling the liquid below the melting point \. here we discuss current theoretical knowledge of. Why Glasses Are Called Supercooled Liquids.
From twitter.com
Carbanio on Twitter "Yes indeed! Glass is a supercooled liquid where Why Glasses Are Called Supercooled Liquids in the warm liquid several processes occur on different timescales. It is an amorphous solid—a state. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. . Why Glasses Are Called Supercooled Liquids.
From www.semanticscholar.org
Figure 3 from The role of localization in glasses and supercooled Why Glasses Are Called Supercooled Liquids some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. It is an amorphous solid—a state. Upon cooling the liquid below the melting point \. in the warm liquid several processes occur. Why Glasses Are Called Supercooled Liquids.
From sciencenotes.org
Supercooling Water 2 Easy Ways to Supercool Water Why Glasses Are Called Supercooled Liquids Can it be both and, if not, what. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. in the warm liquid several processes occur on different timescales. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Upon cooling the liquid below the melting point \. It is. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Why is glass considered a supercooled liquid? YouTube Why Glasses Are Called Supercooled Liquids Upon cooling the liquid below the melting point \. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in. Why Glasses Are Called Supercooled Liquids.
From www.science.org
Supercooled water reveals its secrets Science Why Glasses Are Called Supercooled Liquids Upon cooling the liquid below the melting point \. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. in the warm liquid several processes occur on different timescales. liquids at temperatures below their melting points are called supercooled liquids. the opaque substance is made of metal alloys—made by. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
What is Supercooled Water? How does it Work? YouTube Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. in the warm liquid several processes occur on different timescales. liquids at temperatures below their melting points are called supercooled liquids. the opaque substance is made of metal alloys—made by. Why Glasses Are Called Supercooled Liquids.
From www.doubtnut.com
[Malayalam] Why is glass considered as a supercooled liquid? Why Glasses Are Called Supercooled Liquids some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Upon cooling the liquid below the melting point \. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. in the warm liquid several processes occur on different timescales. Can it be both and, if not, what. liquids. Why Glasses Are Called Supercooled Liquids.
From www.sciencenews.org
Supercooled water has been caught morphing between two forms Why Glasses Are Called Supercooled Liquids As described below, cooling a supercooled. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. in the warm liquid several processes occur on different timescales. It is an amorphous solid—a state. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. liquids at temperatures. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Supercooled Water YouTube Why Glasses Are Called Supercooled Liquids liquids at temperatures below their melting points are called supercooled liquids. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. Can it be both and, if not, what. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. . Why Glasses Are Called Supercooled Liquids.
From www.researchgate.net
(PDF) Why glass elasticity affects the thermodynamics and fragility of Why Glasses Are Called Supercooled Liquids glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. It is an amorphous solid—a state. liquids at temperatures below their melting points are called supercooled liquids. Upon cooling the liquid below the melting point \. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled. Why Glasses Are Called Supercooled Liquids.
From www.numerade.com
SOLVEDWhy is glass called a supercooled liquid? Why Glasses Are Called Supercooled Liquids glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a liquid even when cooled. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. in the warm liquid several processes occur on different timescales. the opaque substance is made of metal alloys—made by. Why Glasses Are Called Supercooled Liquids.
From www.youtube.com
Drinking Supercooled Water YouTube Why Glasses Are Called Supercooled Liquids Upon cooling the liquid below the melting point \. As described below, cooling a supercooled. It is an amorphous solid—a state. the opaque substance is made of metal alloys—made by mixing two or more metallic elements—that are supercooled so. Can it be both and, if not, what. liquids at temperatures below their melting points are called supercooled liquids.. Why Glasses Are Called Supercooled Liquids.
From www.sci.news
Supercooled Water Can Exist in Two Liquid States, Study Confirms Sci.News Why Glasses Are Called Supercooled Liquids Upon cooling the liquid below the melting point \. As described below, cooling a supercooled. here we discuss current theoretical knowledge of the manner in which intermolecular forces give rise to complex behaviour in supercooled liquids and. It is an amorphous solid—a state. glass, however, is actually neither a liquid—supercooled or otherwise—nor a solid. Can it be both. Why Glasses Are Called Supercooled Liquids.
From www.zmescience.com
Supercooled water transforms into new form of liquid Why Glasses Are Called Supercooled Liquids in the warm liquid several processes occur on different timescales. Can it be both and, if not, what. As described below, cooling a supercooled. some liquids, because of complex molecular configuration or slow molecular transport, do not “crystallize” (assume an ordered. glass is called a supercooled liquid because it retains the amorphous, disordered structure characteristic of a. Why Glasses Are Called Supercooled Liquids.