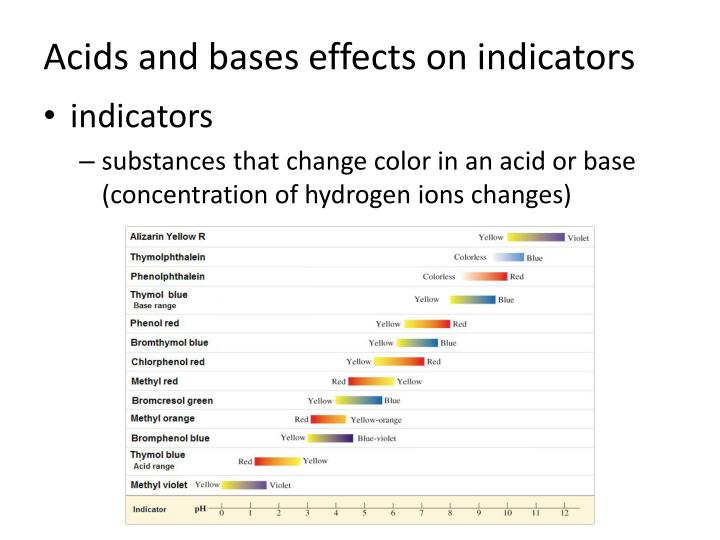
What Are Acids Bases And Indicators . Find out the ph range and pk ind values of common. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Only a small amount of indicator compound is needed to produce a visible color change. Find out the pka or pkb values of common indicators and how to choose the right indicator. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. It is defined as the negative decimal logarithm of the hydrogen ion. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use and calculate the acid dissociation constant.
from www.slideserve.com
It is defined as the negative decimal logarithm of the hydrogen ion. Find out how to select, use and calculate the acid dissociation constant. Find out the ph range and pk ind values of common. Find out the pka or pkb values of common indicators and how to choose the right indicator. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find a table of common indicators, their ph ranges, and how to use them in titrations. Only a small amount of indicator compound is needed to produce a visible color change.
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044
What Are Acids Bases And Indicators It is defined as the negative decimal logarithm of the hydrogen ion. Find out the pka or pkb values of common indicators and how to choose the right indicator. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Only a small amount of indicator compound is needed to produce a visible color change. Find out how to select, use and calculate the acid dissociation constant. It is defined as the negative decimal logarithm of the hydrogen ion. Find out the ph range and pk ind values of common. Find a table of common indicators, their ph ranges, and how to use them in titrations.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. Find out the pka or pkb values of common indicators and how to choose the right indicator. It is defined as the negative decimal logarithm of the hydrogen ion. Only a small amount of indicator compound is needed to produce a visible color change. Find a table of common. What Are Acids Bases And Indicators.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry What Are Acids Bases And Indicators Find out the pka or pkb values of common indicators and how to choose the right indicator. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. It is defined as the negative decimal logarithm of the hydrogen ion. Ph is a logarithmic scale used to specify the acidity or basicity. What Are Acids Bases And Indicators.
From joiwaivrn.blob.core.windows.net
Different Indicators For Acids And Bases at Emily Marshall blog What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use and calculate the acid dissociation constant. Only a small amount of indicator compound is needed to produce a visible color change. Find out the ph range and pk ind values of common. Ph is a logarithmic scale used. What Are Acids Bases And Indicators.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo What Are Acids Bases And Indicators Only a small amount of indicator compound is needed to produce a visible color change. Find out the pka or pkb values of common indicators and how to choose the right indicator. It is defined as the negative decimal logarithm of the hydrogen ion. Find a table of common indicators, their ph ranges, and how to use them in titrations.. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044 What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the ph range and pk ind values of common. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. It is defined as the negative decimal logarithm of the hydrogen ion. See examples of indicators such as. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Only a small amount of indicator compound is needed to produce a visible color change. Find out how to select, use and calculate the acid dissociation constant. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out. What Are Acids Bases And Indicators.
From www.scribd.com
ACIDBASE INDICATORS 211.ppt Acid Ph What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use and calculate the acid dissociation constant. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a. What Are Acids Bases And Indicators.
From www.worksheetsplanet.com
Acid and Bases Differences What Are Acids Bases And Indicators Find out the pka or pkb values of common indicators and how to choose the right indicator. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use and calculate the acid dissociation constant. It is defined as the negative decimal logarithm of the hydrogen ion. See examples of. What Are Acids Bases And Indicators.
From slidetodoc.com
Acids and Bases Properties of Acids and Bases What Are Acids Bases And Indicators Find out the pka or pkb values of common indicators and how to choose the right indicator. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find a table of common indicators, their ph ranges, and how to use them in titrations. Ph is a logarithmic scale used to specify. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID546616 What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Only a small amount of indicator compound is needed to produce a visible color change. Find out how to select, use and calculate the acid dissociation constant. It is defined as the negative decimal logarithm of the hydrogen ion. See examples of indicators such as. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the pka or pkb values of common indicators and how to choose the right indicator. Only a small amount of indicator compound is needed to produce a visible color change. Ph is a logarithmic scale used to specify the acidity or basicity. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation ID2837947 What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Only a small amount of indicator compound is needed to produce a visible color change. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out the pka or pkb values of common indicators and. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. Find out how to select, use and calculate the acid dissociation constant. Find out the pka or pkb values of common indicators and how to choose the right indicator. It is defined as the negative decimal logarithm of the hydrogen ion. Ph is a logarithmic scale used to specify. What Are Acids Bases And Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acids Bases And Indicators See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out the pka or pkb values of common indicators and how to choose the right indicator. Find out the ph range and pk ind values of common. Ph is a logarithmic scale used to specify the acidity or basicity of. What Are Acids Bases And Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. It is defined as the negative decimal logarithm of the hydrogen ion. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Only a small amount of indicator compound is needed to produce a visible. What Are Acids Bases And Indicators.
From freyabaxter.z13.web.core.windows.net
A Chart That Compares Acids And Bases What Are Acids Bases And Indicators Only a small amount of indicator compound is needed to produce a visible color change. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find out the ph range and pk ind values of common. Find a table of common indicators, their ph ranges, and how to use them in titrations. See examples of. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Acids, Bases, and AcidBase Equilibria PowerPoint Presentation What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find a table of common indicators, their ph ranges, and how to use them in titrations. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous. What Are Acids Bases And Indicators.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base What Are Acids Bases And Indicators It is defined as the negative decimal logarithm of the hydrogen ion. Only a small amount of indicator compound is needed to produce a visible color change. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous. What Are Acids Bases And Indicators.
From www.youtube.com
Acid Base Indicators Introduction Acids and Bases Chemistry Practice What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. Find out how to select, use and calculate the acid dissociation constant. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. It is defined as the negative decimal logarithm of the hydrogen ion. Only a small amount of indicator compound is needed to. What Are Acids Bases And Indicators.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator What Are Acids Bases And Indicators See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how to select, use and calculate the acid dissociation constant. Only a small amount of indicator compound is needed to produce a visible color change. Find out the pka or pkb values of common indicators and how to choose. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Conjugate acids and bases PowerPoint Presentation, free download What Are Acids Bases And Indicators See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out the pka or pkb values of common indicators and how to choose the right indicator. Only a small amount of indicator compound is needed to produce a visible color change. Find out how to select, use and calculate the. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free What Are Acids Bases And Indicators Only a small amount of indicator compound is needed to produce a visible color change. Find out how to select, use and calculate the acid dissociation constant. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the pka or pkb values of common indicators and how to choose the right indicator.. What Are Acids Bases And Indicators.
From worksheetcampusbbaya.z13.web.core.windows.net
Explain The Ph Scale Acids And Bases What Are Acids Bases And Indicators Find out the pka or pkb values of common indicators and how to choose the right indicator. Find a table of common indicators, their ph ranges, and how to use them in titrations. Only a small amount of indicator compound is needed to produce a visible color change. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT ACID BASE REACTIONS PowerPoint Presentation, free download ID What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find out the ph range and pk ind values of common. It is defined as the negative decimal logarithm of the hydrogen ion. Find out how to select, use and calculate the acid dissociation constant. See examples of indicators such as litmus, phenolphthalein, and bromophenol. What Are Acids Bases And Indicators.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. Find a table of common indicators, their ph ranges, and how to use them in titrations. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Only a small amount of indicator compound is needed to produce a visible color change. See examples of. What Are Acids Bases And Indicators.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic What Are Acids Bases And Indicators It is defined as the negative decimal logarithm of the hydrogen ion. Find out the ph range and pk ind values of common. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use and calculate the acid dissociation constant. See examples of indicators such as litmus, phenolphthalein, and. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find out how to select, use and calculate the acid dissociation constant. Only a small amount of indicator compound is needed to produce a visible. What Are Acids Bases And Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. It is defined as the negative decimal logarithm of the hydrogen ion. Only a small amount of indicator compound is needed to produce a visible color change. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out how to select, use. What Are Acids Bases And Indicators.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ What Are Acids Bases And Indicators Find a table of common indicators, their ph ranges, and how to use them in titrations. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find out the ph range and pk ind values of. What Are Acids Bases And Indicators.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts What Are Acids Bases And Indicators See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the pka or pkb values of common indicators and how to choose the right indicator. Only a small amount of indicator compound is. What Are Acids Bases And Indicators.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Are Acids Bases And Indicators It is defined as the negative decimal logarithm of the hydrogen ion. Find out how to select, use and calculate the acid dissociation constant. Find out the ph range and pk ind values of common. Only a small amount of indicator compound is needed to produce a visible color change. Find a table of common indicators, their ph ranges, and. What Are Acids Bases And Indicators.
From issr.edu.kh
pKa Values and strengths of Acids and Bases What Are Acids Bases And Indicators Find out the ph range and pk ind values of common. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find out the pka or pkb values of common indicators and how to choose the right indicator. It is defined as the negative decimal logarithm of the hydrogen ion. See examples of indicators such. What Are Acids Bases And Indicators.
From davidswart.wordpress.com
Acids and Bases The Art of Science What Are Acids Bases And Indicators See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the ph range and pk ind values of. What Are Acids Bases And Indicators.
From sciencenotes.org
Facts About Acids and Bases What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. Find a table of common indicators, their ph ranges, and how to use them in titrations. Find out the ph range and pk ind values of common. Only a small amount of indicator compound is needed to produce a visible color change. It is defined. What Are Acids Bases And Indicators.
From pt.slideshare.net
Acids & Bases Indicators What Are Acids Bases And Indicators Ph is a logarithmic scale used to specify the acidity or basicity of aqueous solutions. See examples of indicators such as litmus, phenolphthalein, and bromophenol blue, and how they work on a molecular level. Only a small amount of indicator compound is needed to produce a visible color change. Find out the pka or pkb values of common indicators and. What Are Acids Bases And Indicators.