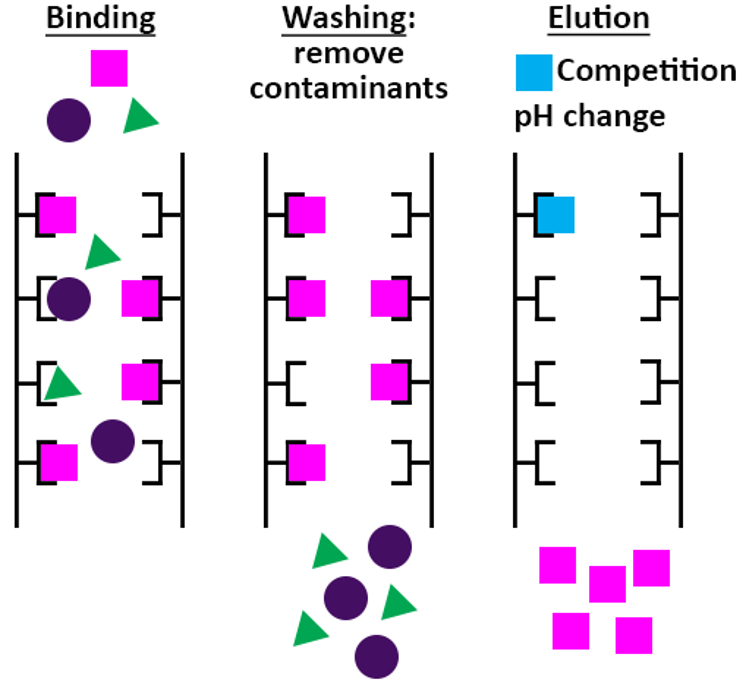
Affinity Chromatography Of Glucose Binding Protein . For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific.
from ar.inspiredpencil.com
Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although.
Affinity Chromatography Diagram
Affinity Chromatography Of Glucose Binding Protein In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography.
From www.numerade.com
You are purifying a glucosebinding protein from a newly discovered Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific. Affinity Chromatography Of Glucose Binding Protein.
From www.selectschoolsupplies.co.uk
Affinity Chromatography of Glucose Binding Proteins Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. For example, many membrane proteins are glycoproteins and can be purified by. Affinity Chromatography Of Glucose Binding Protein.
From jcggdb.jp
sugar binding proteins and their functional analysis Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. In this experiment, students will prepare a seed extract. Affinity Chromatography Of Glucose Binding Protein.
From leon-chapter.blogspot.com
Affinity Chromatography Of Glucose Binding Protein Lab Report 95+ Pages Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. In this experiment, students will prepare a seed. Affinity Chromatography Of Glucose Binding Protein.
From leon-chapter.blogspot.com
Affinity Chromatography Of Glucose Binding Protein Lab Report 95+ Pages Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by.. Affinity Chromatography Of Glucose Binding Protein.
From leon-chapter.blogspot.com
Affinity Chromatography Of Glucose Binding Protein Lab Report 95+ Pages Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by.. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Identification of sugarbinding proteins in osmoticshock fluid from A Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. In this experiment, students will prepare a seed extract from. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Affinity chromatography of schizont proteins on a heparin column. (A Affinity Chromatography Of Glucose Binding Protein The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography is an efficient method to isolate proteins by taking. Affinity Chromatography Of Glucose Binding Protein.
From www.aatbio.com
Affinity Purification AAT Bioquest Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a. Affinity Chromatography Of Glucose Binding Protein.
From discoveringdna.com
Affinity Chromatography of Glucose Binding Proteins Edvotek 277 Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a reversible interaction. Affinity Chromatography Of Glucose Binding Protein.
From journals.sagepub.com
Fluorescence Intensity and LifetimeBased Glucose Sensing Using Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. The most. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Structure of the high affinity Sugar Transport Protein STP10. a The Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose. Affinity Chromatography Of Glucose Binding Protein.
From www.frontiersin.org
Frontiers Recent Advances in LectinBased Affinity Sorbents for Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. The most powerful of these methods. Affinity Chromatography Of Glucose Binding Protein.
From ar.inspiredpencil.com
Affinity Chromatography Apparatus Affinity Chromatography Of Glucose Binding Protein In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. For example, many membrane proteins are glycoproteins and can be purified by. Affinity Chromatography Of Glucose Binding Protein.
From www.cytivalifesciences.com
Affinity chromatography for antibody protein purification Cytiva Affinity Chromatography Of Glucose Binding Protein In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates,. Affinity Chromatography Of Glucose Binding Protein.
From www.neuromics.com
Protein Affinity Chromatography Affinity Chromatography Of Glucose Binding Protein The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. In this experiment, students will prepare a seed extract. Affinity Chromatography Of Glucose Binding Protein.
From www.caframolabsolutions.com
Protein Affinity Chromatography Caframo Lab Solutions Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. The most powerful of these methods is affinity chromatography, also called affinity. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
(PDF) Amylose Affinity Chromatography of MaltoseBinding Protein Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography is an efficient method to isolate proteins by. Affinity Chromatography Of Glucose Binding Protein.
From www.eurekalert.org
Regulation of ProteinLigand Binding Affinity EurekAlert! Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by.. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Binding modes of glucose and various inhibitors to human SGLT2 Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand.. Affinity Chromatography Of Glucose Binding Protein.
From ar.inspiredpencil.com
Affinity Chromatography Animation Affinity Chromatography Of Glucose Binding Protein The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. In this experiment, students will prepare a. Affinity Chromatography Of Glucose Binding Protein.
From suvduush007.blogspot.com
Affinity Chromatography Of Glucose Binding Protein Lab Report 24+ Pages Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. For example, many membrane proteins are glycoproteins and. Affinity Chromatography Of Glucose Binding Protein.
From www.slideshare.net
Protein Purification Hjp Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography. Affinity Chromatography Of Glucose Binding Protein.
From ar.inspiredpencil.com
Affinity Chromatography Apparatus Affinity Chromatography Of Glucose Binding Protein Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography of glycoproteins is currently. Affinity Chromatography Of Glucose Binding Protein.
From www.numerade.com
SOLVED You are purifying a glucosebinding protein from a newly Affinity Chromatography Of Glucose Binding Protein In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. For example, many membrane proteins are glycoproteins and can be purified by lectin. Affinity Chromatography Of Glucose Binding Protein.
From hxejouhoi.blob.core.windows.net
Affinity Chromatography Binding Buffer at Jesus Conley blog Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose. Affinity Chromatography Of Glucose Binding Protein.
From mavink.com
Affinity Chromatography Diagram Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. In this experiment, students will prepare a seed extract from. Affinity Chromatography Of Glucose Binding Protein.
From www.slideserve.com
PPT Protein Purification PowerPoint Presentation, free download ID Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography separates proteins on the. Affinity Chromatography Of Glucose Binding Protein.
From www.semanticscholar.org
Figure 1 from 3 Protein Purification by Affinity Chromatography Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by.. Affinity Chromatography Of Glucose Binding Protein.
From ar.inspiredpencil.com
Affinity Chromatography Diagram Affinity Chromatography Of Glucose Binding Protein Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the. Affinity Chromatography Of Glucose Binding Protein.
From www.goldbio.com
How Column Chromatography Works to Separate Proteins GoldBio Affinity Chromatography Of Glucose Binding Protein The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography is an efficient method to isolate proteins by taking advantage of. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Protein affinity chromatography. Extract proteins are passed over a Affinity Chromatography Of Glucose Binding Protein In this experiment, students will prepare a seed extract from jack bean meal, fractionate the extract by affinity chromatography, and elute the bound glucose binding protein. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. For example, many membrane. Affinity Chromatography Of Glucose Binding Protein.
From leon-chapter.blogspot.com
Affinity Chromatography Of Glucose Binding Protein Lab Report 95+ Pages Affinity Chromatography Of Glucose Binding Protein The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography separates proteins on the basis of a reversible interaction between a protein (or group of proteins) and a specific ligand. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. In this experiment, students. Affinity Chromatography Of Glucose Binding Protein.
From www.researchgate.net
Affinity chromatography of hepatocyte plasma membrane proteins Affinity Chromatography Of Glucose Binding Protein For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography of glycoproteins is currently conducted with immobilized lectins or boronates, although. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. Affinity chromatography is an efficient method to isolate proteins by taking. Affinity Chromatography Of Glucose Binding Protein.
From thechemistrynotes.com
Affinity chromatography Affinity Chromatography Of Glucose Binding Protein Affinity chromatography is an efficient method to isolate proteins by taking advantage of their affinities for specific. The most powerful of these methods is affinity chromatography, also called affinity purification, whereby the protein of interest is purified by. For example, many membrane proteins are glycoproteins and can be purified by lectin affinity chromatography. Affinity chromatography of glycoproteins is currently conducted. Affinity Chromatography Of Glucose Binding Protein.