Energy Levels Chart . chapter 4, lesson 3: Each has its own specific energy level and properties. because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. the number of electrons in each energy level is displayed on the periodic table. what are energy levels? The number of elements in each row shows how many electrons it takes to fill. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. there are multiple orbitals within an atom. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. • the electrons surrounding an atom are located in. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. Because each orbital is different, they are.
from www.vedantu.com
the number of electrons in each energy level is displayed on the periodic table. Each has its own specific energy level and properties. there are multiple orbitals within an atom. • the electrons surrounding an atom are located in. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. chapter 4, lesson 3: what are energy levels? because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. Because each orbital is different, they are. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
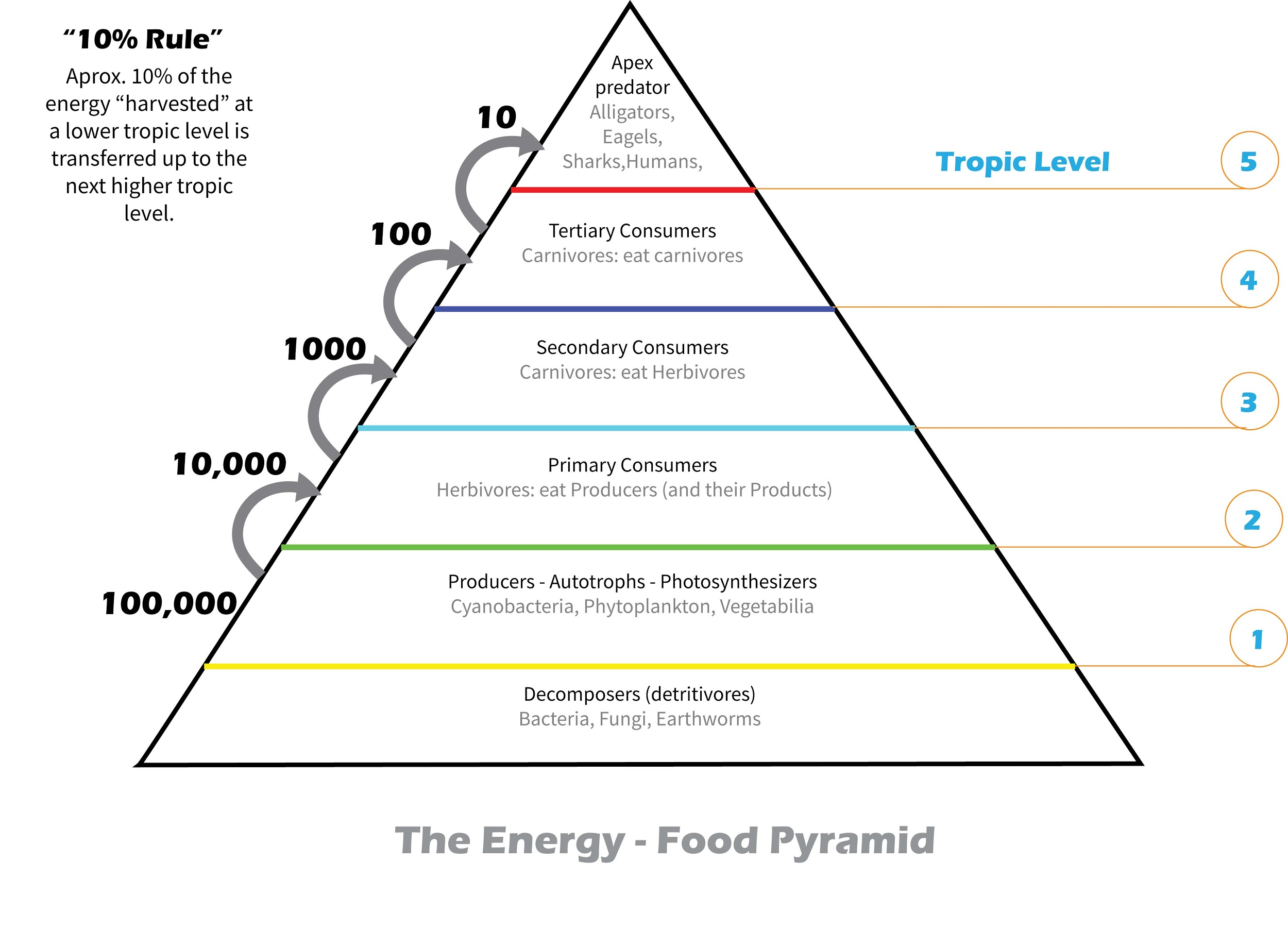
Explain the energy pyramid.
Energy Levels Chart Each has its own specific energy level and properties. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. chapter 4, lesson 3: in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. • the electrons surrounding an atom are located in. the number of electrons in each energy level is displayed on the periodic table. what are energy levels? because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. Each has its own specific energy level and properties. The number of elements in each row shows how many electrons it takes to fill. there are multiple orbitals within an atom. Because each orbital is different, they are.
From animalia-life.club
Electron Energy Levels Chart Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. there are multiple orbitals within an atom. Each has its own specific energy level and properties. chapter 4, lesson 3: the number of electrons in each energy level is displayed on the periodic table. because its average distance. Energy Levels Chart.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. there are multiple orbitals within an atom. Because each orbital is different, they are. The number of elements in each row shows how many electrons it takes to fill. what are energy levels? the number of electrons in each. Energy Levels Chart.
From learningcampusdustin.z4.web.core.windows.net
Electrons In Energy Levels Chart Energy Levels Chart in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. there are multiple orbitals within an atom. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. Because each orbital is different, they are. . Energy Levels Chart.
From mungfali.com
Human Body Energy Chart Energy Levels Chart each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. what are energy levels? in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.. Energy Levels Chart.
From www.researchgate.net
a Energy levels of ITO, PEDOTPSS, PTB7, PC71BM, PBDBT, ITIC Energy Levels Chart because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. Each has its own specific energy level and properties. Because each orbital is different, they are. what are energy levels? in this section we will discuss the energy level. Energy Levels Chart.
From partdiagramtobdeoi.z13.web.core.windows.net
Energy Diagram For Hydrogen Atom Energy Levels Chart chapter 4, lesson 3: there are multiple orbitals within an atom. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. what are energy levels? the number of electrons in each energy level is displayed on the. Energy Levels Chart.
From animalia-life.club
Electron Energy Levels Chart Energy Levels Chart The number of elements in each row shows how many electrons it takes to fill. chapter 4, lesson 3: Because each orbital is different, they are. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. each element has a unique set of energy levels, and so the frequencies at. Energy Levels Chart.
From physics.stackexchange.com
quantum mechanics How to understand the hydrogen energy level and its Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. chapter 4, lesson 3: The number of elements in each row shows how many electrons it takes to fill. Each has its own specific energy level and properties. because its average distance from the nucleus determines the energy of an. Energy Levels Chart.
From circuitdiagramgyte.z22.web.core.windows.net
Draw Energy Level Diagram Of Hydrogen Atom Energy Levels Chart each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. what are energy levels? the number of electrons in each energy level is displayed on the periodic table. Energy levels (also called electron shells) are fixed distances from the. Energy Levels Chart.
From www.physics.udel.edu
Electron Energy Levels Energy Levels Chart The number of elements in each row shows how many electrons it takes to fill. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. • the electrons surrounding an atom are located in. each element has a unique set of energy levels, and so the frequencies at which it absorbs. Energy Levels Chart.
From goe.ac
Energy Chart Images GoE Energy Levels Chart Because each orbital is different, they are. the number of electrons in each energy level is displayed on the periodic table. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits. Energy Levels Chart.
From wiredataeidzj.z21.web.core.windows.net
Energy Diagram For Hydrogen Atom Energy Levels Chart in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. the number of electrons. Energy Levels Chart.
From www.chemicals.co.uk
A Level Chemistry Revision Chemistry Periodicity Energy Levels Chart Because each orbital is different, they are. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. what are energy levels? the number of electrons in each energy level is displayed on the periodic table. Energy levels (also called electron shells) are. Energy Levels Chart.
From scientifictutor.org
Chem Energy Levels Scientific Tutor Energy Levels Chart Because each orbital is different, they are. • the electrons surrounding an atom are located in. chapter 4, lesson 3: The number of elements in each row shows how many electrons it takes to fill. Each has its own specific energy level and properties. because its average distance from the nucleus determines the energy of an electron, each. Energy Levels Chart.
From turningthecornerllc.com
How To Use Energy Leadership Leadership Energy Turning The Corner Energy Levels Chart Each has its own specific energy level and properties. the number of electrons in each energy level is displayed on the periodic table. Because each orbital is different, they are. • the electrons surrounding an atom are located in. there are multiple orbitals within an atom. Energy levels (also called electron shells) are fixed distances from the nucleus. Energy Levels Chart.
From wiredatalunarlev5w.z21.web.core.windows.net
Sni3 Electron Geometry And Orbital Diagram Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. the number of electrons in each energy level is displayed on the periodic table. chapter 4, lesson 3: Because each orbital is different, they are. there are multiple orbitals within an atom. each element has a unique set. Energy Levels Chart.
From www.howdens.com
Appliance Energy Ratings Guide Buying Guide Howdens Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. the number of electrons in each energy level is displayed on the periodic table. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Each. Energy Levels Chart.
From www.vedantu.com
Explain the energy pyramid. Energy Levels Chart chapter 4, lesson 3: Each has its own specific energy level and properties. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. The number of elements in each row shows how many electrons it takes to fill. because. Energy Levels Chart.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.5.2 Energy Level Diagrams翰林国际教育 Energy Levels Chart what are energy levels? because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. Because each orbital is different, they are. Each has its own specific energy level and properties. each element has a unique set of energy levels,. Energy Levels Chart.
From naomiwade.z21.web.core.windows.net
Electron Energy Levels Chart Energy Levels Chart in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. there are multiple orbitals. Energy Levels Chart.
From www.departmentofproduct.com
How to Structure your Day Using Energy Levels as a Product Manager Energy Levels Chart Each has its own specific energy level and properties. The number of elements in each row shows how many electrons it takes to fill. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Energy levels (also called electron shells) are fixed distances from. Energy Levels Chart.
From scientifictutor.org
Chem Energy Levels Scientific Tutor Energy Levels Chart chapter 4, lesson 3: there are multiple orbitals within an atom. • the electrons surrounding an atom are located in. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. the number of electrons in each energy level is displayed on. Energy Levels Chart.
From quizconsectary.z21.web.core.windows.net
How To Find Energy Levels Energy Levels Chart what are energy levels? Each has its own specific energy level and properties. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. • the electrons surrounding an atom are located in. Because each orbital is different, they are. because its average distance from the nucleus determines the energy of. Energy Levels Chart.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons Energy Levels Chart what are energy levels? Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. Because each orbital is different, they are. • the electrons surrounding an atom are located in. The number of elements in each row shows how many electrons it takes to fill. the number of electrons in. Energy Levels Chart.
From animalia-life.club
Electron Energy Levels Chart Energy Levels Chart • the electrons surrounding an atom are located in. chapter 4, lesson 3: there are multiple orbitals within an atom. Each has its own specific energy level and properties. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. the number. Energy Levels Chart.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Energy Levels Chart in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. • the electrons surrounding an atom are located in. there are multiple orbitals within an atom. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons. Energy Levels Chart.
From www.theagendaperiod.com
Energetic Levels Throughout Your Cycle The Agenda. Energy Levels Chart chapter 4, lesson 3: what are energy levels? • the electrons surrounding an atom are located in. Because each orbital is different, they are. Each has its own specific energy level and properties. The number of elements in each row shows how many electrons it takes to fill. there are multiple orbitals within an atom. in. Energy Levels Chart.
From skintots.com
What Is An Energy Level Apex? Energy Levels Chart • the electrons surrounding an atom are located in. The number of elements in each row shows how many electrons it takes to fill. because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. chapter 4, lesson 3: each. Energy Levels Chart.
From www.slideserve.com
PPT Chapter 40 PowerPoint Presentation, free download ID5785191 Energy Levels Chart there are multiple orbitals within an atom. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. chapter 4, lesson 3: Each has its own specific energy level and properties. • the electrons surrounding an atom are located in. Energy levels (also. Energy Levels Chart.
From schematicmaxeycreaghs.z21.web.core.windows.net
Energy Diagram For An Exothermic Reaction Energy Levels Chart the number of electrons in each energy level is displayed on the periodic table. there are multiple orbitals within an atom. Each has its own specific energy level and properties. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. chapter. Energy Levels Chart.
From naomiwade.z21.web.core.windows.net
Electrons In Energy Levels Chart Energy Levels Chart Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. The number of elements in each row shows how many electrons it takes. Energy Levels Chart.
From members.believeperform.com
10 ways to manage your energy levels throughout the day Energy Levels Chart what are energy levels? Because each orbital is different, they are. each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may. . Energy Levels Chart.
From wirepartnemertines.z5.web.core.windows.net
Diagram Of Hydrogen Atom Energy Levels Chart what are energy levels? each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. there are multiple orbitals within an atom. Because each orbital is different, they are. in this section we will discuss the energy level of. Energy Levels Chart.
From www.slideserve.com
PPT Matter and more PowerPoint Presentation, free download ID2537776 Energy Levels Chart Because each orbital is different, they are. The number of elements in each row shows how many electrons it takes to fill. because its average distance from the nucleus determines the energy of an electron, each atomic orbital with a given set of quantum numbers has a particular energy associated. each element has a unique set of energy. Energy Levels Chart.
From imgflip.com
Energy Levels Imgflip Energy Levels Chart each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the. there are multiple orbitals within an atom. • the electrons surrounding an atom are located in. Energy levels (also called electron shells) are fixed distances from the nucleus of an. Energy Levels Chart.