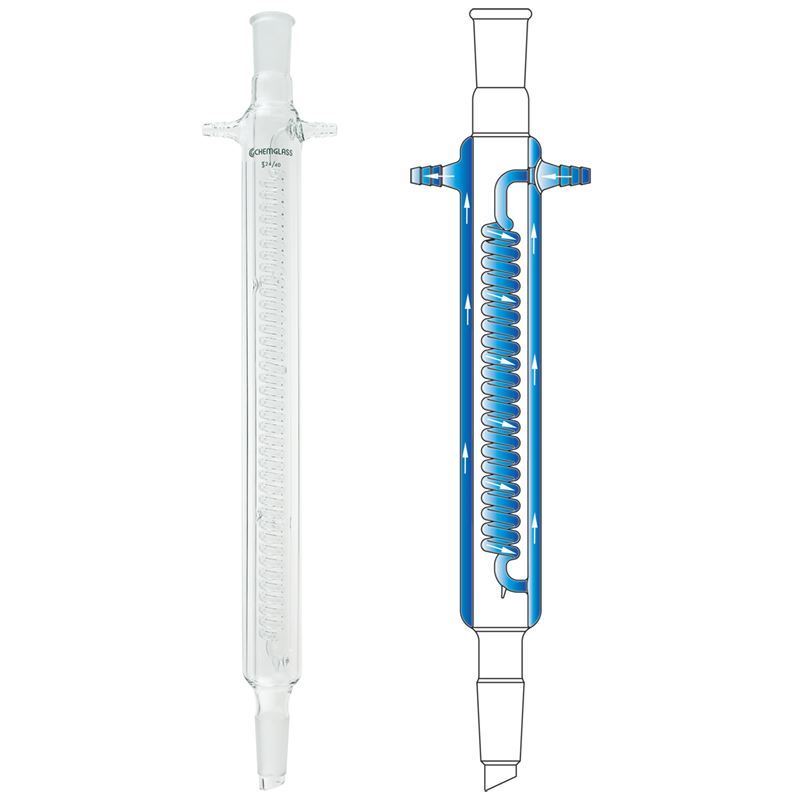
Reflux And Condenser Reaction . Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. So, you need to drive a chemical reaction by heating it at reflux. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. A reflux setup is analogous. You will need to heat the reaction at the boiling point of your solvent(s). In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. What types of reactants can be used in reflux experiment? The temperature of the reaction remains constant. The condensation of the vapors above the reaction returns to the flask. Example reactions where heating under reflux could be used include:
from chemglass.com
Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. So, you need to drive a chemical reaction by heating it at reflux. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. The condensation of the vapors above the reaction returns to the flask. The temperature of the reaction remains constant. What types of reactants can be used in reflux experiment? A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. You will need to heat the reaction at the boiling point of your solvent(s). Example reactions where heating under reflux could be used include:
CG1215A CONDENSERS, REFLUX Chemglass Life Sciences
Reflux And Condenser Reaction The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. So, you need to drive a chemical reaction by heating it at reflux. What types of reactants can be used in reflux experiment? The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. The temperature of the reaction remains constant. Example reactions where heating under reflux could be used include: In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. A reflux setup is analogous. The condensation of the vapors above the reaction returns to the flask. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. You will need to heat the reaction at the boiling point of your solvent(s).
From www.numerade.com
SOLVED Conditions air reflux condenser thermometer clamp clamps Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas. Reflux And Condenser Reaction.
From www.dreamstime.com
Lab Apparatus for Reflux, Reflux is a Technique Involving the Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. What types of reactants can be used in reflux experiment? Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. Once the reflux condenser has. Reflux And Condenser Reaction.
From www.researchgate.net
Schematic of reflux setup. Download Scientific Diagram Reflux And Condenser Reaction The temperature of the reaction remains constant. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. In chemistry, reflux involves heating a chemical reaction. Reflux And Condenser Reaction.
From quizlet.com
Distillation and Reflux Diagram Quizlet Reflux And Condenser Reaction In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and. Reflux And Condenser Reaction.
From www.hotelpresident.ci
Reflux Condenser Diagram hotelpresident.ci Reflux And Condenser Reaction Example reactions where heating under reflux could be used include: Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. So, you need to drive. Reflux And Condenser Reaction.
From schematicdataweals77.z13.web.core.windows.net
Reflux Apparatus Diagram A Level Chemistry Reflux And Condenser Reaction You will need to heat the reaction at the boiling point of your solvent(s). The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. So, you need to drive a chemical reaction by heating it at reflux. The production of a carboxylic acid from a primary. Reflux And Condenser Reaction.
From www.devasthalihotels.com
חברותי עצה גלגל שיניים reflux condenser purpose כותנה מזיקים שעועית Reflux And Condenser Reaction You will need to heat the reaction at the boiling point of your solvent(s). The temperature of the reaction remains constant. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using. Reflux And Condenser Reaction.
From www.youtube.com
How to set up a reflux reaction using the Findenser waterless glass Reflux And Condenser Reaction A reflux setup is analogous. In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. The condensation of the vapors above the reaction returns to the. Reflux And Condenser Reaction.
From chemglass.com
CG1215A CONDENSERS, REFLUX Chemglass Life Sciences Reflux And Condenser Reaction You will need to heat the reaction at the boiling point of your solvent(s). Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. So, you need to drive a chemical reaction by heating it at reflux. What types of reactants can be used in reflux experiment? A reflux. Reflux And Condenser Reaction.
From www.researchgate.net
The scheme of the reaction setup. 1 mechanical stirrer; 2 dropper Reflux And Condenser Reaction In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. A reflux setup is analogous. So, you need to drive a chemical reaction by heating it at reflux. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The. Reflux And Condenser Reaction.
From www.thestudentroom.co.uk
AS Biology Chemistry The Student Room Reflux And Condenser Reaction What types of reactants can be used in reflux experiment? So, you need to drive a chemical reaction by heating it at reflux. A reflux setup is analogous. In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. The principle of the reflux operation is to safely provide a controlled. Reflux And Condenser Reaction.
From www.slideserve.com
PPT Heating with a reflux condenser PowerPoint Presentation ID6836735 Reflux And Condenser Reaction The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. So, you need to drive a chemical reaction by heating it at reflux. The temperature of the reaction remains constant. In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. The principle of. Reflux And Condenser Reaction.
From chemistry.stackexchange.com
organic chemistry What is "heating under reflux"? Chemistry Stack Reflux And Condenser Reaction So, you need to drive a chemical reaction by heating it at reflux. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. What types of reactants can be. Reflux And Condenser Reaction.
From chem.libretexts.org
1.4K Reflux Chemistry LibreTexts Reflux And Condenser Reaction The temperature of the reaction remains constant. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. What types of reactants can be used in reflux experiment? So, you need to drive a chemical reaction by heating it at reflux. Once the reflux condenser has been cycled onto the schlenk line, backfill with. Reflux And Condenser Reaction.
From www.hoddereducationmagazines.com
Heating under reflux Hodder Education Magazines Reflux And Condenser Reaction The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. What types of reactants can be used in reflux experiment? A reflux setup is analogous. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. A reflux setup (figure 1.58) allows for. Reflux And Condenser Reaction.
From www.sciencephoto.com
Reflux heating using an electric mantle Stock Image T875/1274 Reflux And Condenser Reaction The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under. Reflux And Condenser Reaction.
From chem.libretexts.org
1.4K Reflux Chemistry LibreTexts Reflux And Condenser Reaction A reflux setup is analogous. The condensation of the vapors above the reaction returns to the flask. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. Example reactions where heating under reflux could be used include: You will need to heat the reaction at the boiling point of your solvent(s). The temperature. Reflux And Condenser Reaction.
From skc13chem.blogspot.com
SKC year 13 Chemistry Organic chemistry Reflux and distillation Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. Example reactions where heating under reflux could be used include: Reflux involves heating the chemical reaction for a specific. Reflux And Condenser Reaction.
From www.savemyexams.com
Oxidation of Alcohols SL IB Chemistry Revision Notes 2025 Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. You will need to heat the reaction at the boiling point of. Reflux And Condenser Reaction.
From www.thesciencehive.co.uk
Organic Synthesis* — the science sauce Reflux And Condenser Reaction What types of reactants can be used in reflux experiment? The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. The temperature of the reaction. Reflux And Condenser Reaction.
From www.thevespiary.org
Lycaeum > Leda > Diagram of reaction vessel with reflux condenser. Reflux And Condenser Reaction The condensation of the vapors above the reaction returns to the flask. You will need to heat the reaction at the boiling point of your solvent(s). A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. The production of a carboxylic acid from a primary alcohol using acidified potassium. Reflux And Condenser Reaction.
From www.coursehero.com
[Solved] 1.In a reflux set up in an organic chemistry lab what happens Reflux And Condenser Reaction You will need to heat the reaction at the boiling point of your solvent(s). In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. A reflux setup is analogous. The condensation of the vapors above the reaction returns to the flask. The principle of the reflux operation is to safely. Reflux And Condenser Reaction.
From www.youtube.com
A Brief Introduction to Refluxing YouTube Reflux And Condenser Reaction Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. You will need to heat the reaction at the boiling point of your solvent(s). A. Reflux And Condenser Reaction.
From www.alamy.com
reflux condenser on a water bath Stock Photo 133815 Alamy Reflux And Condenser Reaction You will need to heat the reaction at the boiling point of your solvent(s). The condensation of the vapors above the reaction returns to the flask. In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert. Reflux And Condenser Reaction.
From chemglass.com
CG1213 CONDENSERS, REFLUX Chemglass Life Sciences Reflux And Condenser Reaction The condensation of the vapors above the reaction returns to the flask. A reflux setup (figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original flask. You will need to heat the reaction at the boiling point of your solvent(s). The production of a carboxylic acid from a primary alcohol using acidified potassium. Reflux And Condenser Reaction.
From www.youtube.com
Reaction reflux using a waterless condenser YouTube Reflux And Condenser Reaction A reflux setup is analogous. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The temperature of the reaction remains constant. What types of reactants can be used in reflux experiment? In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it. Reflux And Condenser Reaction.
From pharmaguides.in
2.2 Reflux Condenser Principle, Diagram In Details Reflux And Condenser Reaction What types of reactants can be used in reflux experiment? Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The condensation of the vapors above the reaction returns to the flask. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach. Reflux And Condenser Reaction.
From stock.adobe.com
Laboratory setup for reflux reactions. With a Dimroth condenser. Round Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. The condensation of the vapors above the reaction returns to the flask. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive.. Reflux And Condenser Reaction.
From tophat.com
Organic Chemistry Laboratory Techniques [For Standard and Microscale Reflux And Condenser Reaction In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. So, you need to drive a chemical reaction by heating it at reflux. The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. Reflux involves heating the chemical reaction for a specific amount. Reflux And Condenser Reaction.
From favpng.com
Water Condenser Condensation Reflux Laboratory, PNG, 1093x1085px, Water Reflux And Condenser Reaction So, you need to drive a chemical reaction by heating it at reflux. A reflux setup is analogous. What types of reactants can be used in reflux experiment? Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. A reflux setup (figure 1.58) allows for liquid to boil and. Reflux And Condenser Reaction.
From www.slideserve.com
PPT Substitution Reactions 1 The Sn 2 Reaction The Synthesis of 1 Reflux And Condenser Reaction Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. Example reactions where heating under reflux could be used include: The production of a carboxylic. Reflux And Condenser Reaction.
From skc13chem.blogspot.hk
SKC year 13 Chemistry Organic chemistry Reflux and distillation Reflux And Condenser Reaction The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. Once the reflux condenser has been cycled onto the schlenk line, backfill with inert gas and attach to the schlenk flask under a positive. So, you need to drive a chemical reaction by heating it at. Reflux And Condenser Reaction.
From www.pngegg.com
Reflux Condenser Chemistry Chemical synthesis Roundbottom flask Reflux And Condenser Reaction What types of reactants can be used in reflux experiment? The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. The condensation of the vapors above the reaction returns to the flask. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid.. Reflux And Condenser Reaction.
From themasterchemistry.com
What Is Reflux In Chemistry? A Detailed Guide Reflux And Condenser Reaction Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid. A reflux setup is analogous. Example reactions where heating under reflux could be used include: You will need to heat the reaction at the boiling point of your solvent(s). So, you need to drive a chemical reaction by heating. Reflux And Condenser Reaction.
From telgurus.co.uk
What is reflux in chemistry? Why is it used? Chemistry questionnaire. Reflux And Condenser Reaction The production of a carboxylic acid from a primary alcohol using acidified potassium dichromate the production of. The principle of the reflux operation is to safely provide a controlled amount of heat to the reaction, while ensuring that the solvent does. Example reactions where heating under reflux could be used include: Reflux involves heating the chemical reaction for a specific. Reflux And Condenser Reaction.