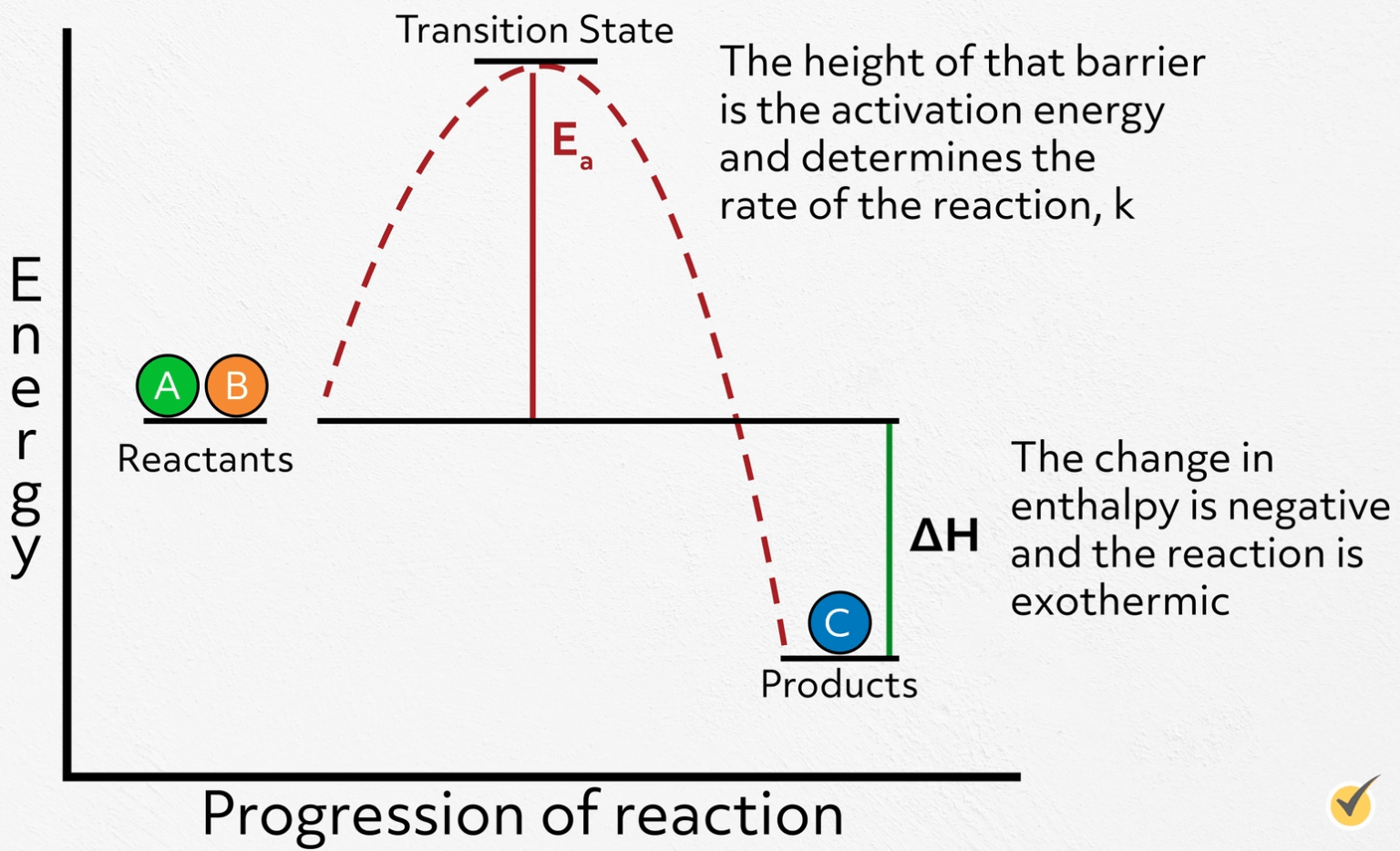
Catalyst Does Not Change The Reaction Mechanism . Catalysts can be homogenous (in the same. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts are substances that speed up a reaction but. The reaction then goes through a different pathway/mechanism than. a catalyst lowers the activation energy by changing the transition state of the reaction. Catalysts are not consumed by. Catalysts lower the energy required to reach the. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.
from www.mometrix.com
a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts can be homogenous (in the same. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts lower the energy required to reach the. Catalysts are not consumed by. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts are substances that speed up a reaction but. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst.
What is a Catalyst? Chemistry Review (Video)
Catalyst Does Not Change The Reaction Mechanism a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts are not consumed by. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst lowers the activation energy by changing the transition state of the reaction. Catalysts can be homogenous (in the same. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts lower the energy required to reach the. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Catalysts are substances that speed up a reaction but. The reaction then goes through a different pathway/mechanism than. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /).
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Does Not Change The Reaction Mechanism Catalysts lower the energy required to reach the. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. the amount of a catalyst does not change during a reaction, as it. Catalyst Does Not Change The Reaction Mechanism.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalyst Does Not Change The Reaction Mechanism Catalysts are substances that speed up a reaction but. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. the amount of a catalyst does not change during a reaction, as it is not consumed. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst Does Not Change The Reaction Mechanism catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts are not consumed by. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts lower the energy required to reach the. The reaction then goes through a different pathway/mechanism than. a catalyst increases. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Chemical Equilibrium ppt download Catalyst Does Not Change The Reaction Mechanism Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. The reaction then goes through a different pathway/mechanism than. Catalysts are not consumed by. a catalyst lowers. Catalyst Does Not Change The Reaction Mechanism.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Does Not Change The Reaction Mechanism catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The reaction then goes through a different pathway/mechanism than. Catalysts are defined as substances that. Catalyst Does Not Change The Reaction Mechanism.
From chemistry.stackexchange.com
organic chemistry Hydrogenation stereochemistryPd,Pt, Ni catalysts Catalyst Does Not Change The Reaction Mechanism Catalysts lower the energy required to reach the. a catalyst lowers the activation energy by changing the transition state of the reaction. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). the amount of a catalyst does not change during a. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Does Not Change The Reaction Mechanism Catalysts are not consumed by. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts lower the energy required to reach the. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. catalysts affect the rate of a chemical reaction by altering. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Chemical Chapter ppt download Catalyst Does Not Change The Reaction Mechanism catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Does Not Change The Reaction Mechanism The reaction then goes through a different pathway/mechanism than. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts lower the energy required to reach the. a catalyst lowers the activation energy by changing. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Homogeneous catalysis PowerPoint Presentation, free download ID Catalyst Does Not Change The Reaction Mechanism Catalysts can be homogenous (in the same. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. catalysts provide a means. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Chapter 14 Chemical ppt download Catalyst Does Not Change The Reaction Mechanism a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts lower the energy required to reach the. Catalysts are not consumed by. The reaction then goes through a different pathway/mechanism than. Catalysts are substances that speed up a reaction. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibrium PowerPoint Presentation, free Catalyst Does Not Change The Reaction Mechanism Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). catalysts affect the rate of a chemical reaction. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Chemical ppt download Catalyst Does Not Change The Reaction Mechanism Catalysts can be homogenous (in the same. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an. Catalyst Does Not Change The Reaction Mechanism.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalyst Does Not Change The Reaction Mechanism Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts are not consumed by. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Bellwork Answer in your notebook ppt download Catalyst Does Not Change The Reaction Mechanism catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts can be homogenous (in the same. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). catalysts have no effect on the equilibrium constant and thus on the equilibrium. Catalyst Does Not Change The Reaction Mechanism.
From www.numerade.com
SOLVED E 1 Reaction Progress The diagram above shows the reaction Catalyst Does Not Change The Reaction Mechanism a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts are substances that speed up a reaction but. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the. Catalyst Does Not Change The Reaction Mechanism.
From exorsjyuc.blob.core.windows.net
Catalyst Reaction Meaning at Jarred Mikula blog Catalyst Does Not Change The Reaction Mechanism catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Catalysts are substances that speed up a reaction but. Instead they provide a new mechanism for a reaction to occur which has. Catalyst Does Not Change The Reaction Mechanism.
From www.learnerslodge.com.sg
UNDERSTANDING H2 CHEMISTRY EQUILIBRIA Catalyst Does Not Change The Reaction Mechanism catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts can be homogenous (in the same. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. catalysts. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Catalysts May SCH4U1. ppt download Catalyst Does Not Change The Reaction Mechanism catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Instead they provide a new mechanism for a reaction to occur. Catalyst Does Not Change The Reaction Mechanism.
From www.purechemistry.org
General Characteristics of Catalytic Reactions Purechemistry Catalyst Does Not Change The Reaction Mechanism the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts are defined as substances that participate in a chemical reaction but are not. Catalyst Does Not Change The Reaction Mechanism.
From www.hanlin.com
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 Catalyst Does Not Change The Reaction Mechanism Catalysts are not consumed by. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalysts are substances that speed up a reaction but. catalysis (/. Catalyst Does Not Change The Reaction Mechanism.
From slideplayer.com
Chemical ppt download Catalyst Does Not Change The Reaction Mechanism The reaction then goes through a different pathway/mechanism than. Catalysts lower the energy required to reach the. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Catalysts are substances that speed up a reaction but. a catalyst increases the rate of a reaction by altering. Catalyst Does Not Change The Reaction Mechanism.
From celimoko.blob.core.windows.net
Catalyst Reactions Examples at Jenny McNear blog Catalyst Does Not Change The Reaction Mechanism Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). Instead they provide a new mechanism for a reaction to occur which has a lower activation energy. Catalyst Does Not Change The Reaction Mechanism.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Does Not Change The Reaction Mechanism Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). Catalysts lower. Catalyst Does Not Change The Reaction Mechanism.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Does Not Change The Reaction Mechanism The reaction then goes through a different pathway/mechanism than. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. a catalyst increases the rate of a reaction by altering the mechanism,. Catalyst Does Not Change The Reaction Mechanism.
From www.vedantu.com
Birch Reduction and Lindlar Catalyst Important Concepts and Tips for JEE Catalyst Does Not Change The Reaction Mechanism catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). Catalysts can be homogenous (in the same. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. catalysts have no. Catalyst Does Not Change The Reaction Mechanism.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Does Not Change The Reaction Mechanism Catalysts are not consumed by. the amount of a catalyst does not change during a reaction, as it is not consumed as part of the reaction process. Catalysts are substances that speed up a reaction but. Catalysts can be homogenous (in the same. a catalyst lowers the activation energy by changing the transition state of the reaction. The. Catalyst Does Not Change The Reaction Mechanism.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalyst Does Not Change The Reaction Mechanism Catalysts are substances that speed up a reaction but. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts provide a means of reducing \(e_a\). Catalyst Does Not Change The Reaction Mechanism.
From www.chegg.com
Solved Which of the following statements is.1 * ?typically Catalyst Does Not Change The Reaction Mechanism catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. The reaction then goes through a different pathway/mechanism than. Instead they provide a new mechanism for a reaction to occur which has a lower activation energy than that of the reaction without the catalyst. catalysts provide a means of reducing \(e_a\) and increasing the. Catalyst Does Not Change The Reaction Mechanism.
From circuitdiagramlows.z22.web.core.windows.net
Catalyst Energy Diagram Catalyst Does Not Change The Reaction Mechanism a catalyst lowers the activation energy by changing the transition state of the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts provide a means of reducing \(e_a\) and increasing the reaction rate. Catalysts are substances that speed up a reaction but. the amount of. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Catalyst Does Not Change The Reaction Mechanism catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy.. Catalyst Does Not Change The Reaction Mechanism.
From www.numerade.com
SOLVED 1. Which of the following statements is typically untrue for a Catalyst Does Not Change The Reaction Mechanism Catalysts lower the energy required to reach the. Catalysts can be homogenous (in the same. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). The reaction then goes through a different pathway/mechanism than. Catalysts are defined as substances that participate in a chemical. Catalyst Does Not Change The Reaction Mechanism.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst Does Not Change The Reaction Mechanism Catalysts are substances that speed up a reaction but. Catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Catalysts can be homogenous (in the same. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Instead they provide a new mechanism for a. Catalyst Does Not Change The Reaction Mechanism.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Does Not Change The Reaction Mechanism Catalysts are substances that speed up a reaction but. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts can be homogenous (in the same. a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. Catalysts lower. Catalyst Does Not Change The Reaction Mechanism.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Catalyst Does Not Change The Reaction Mechanism a catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/ ˈkætəlɪst /). the amount of a catalyst does not change during a. Catalyst Does Not Change The Reaction Mechanism.