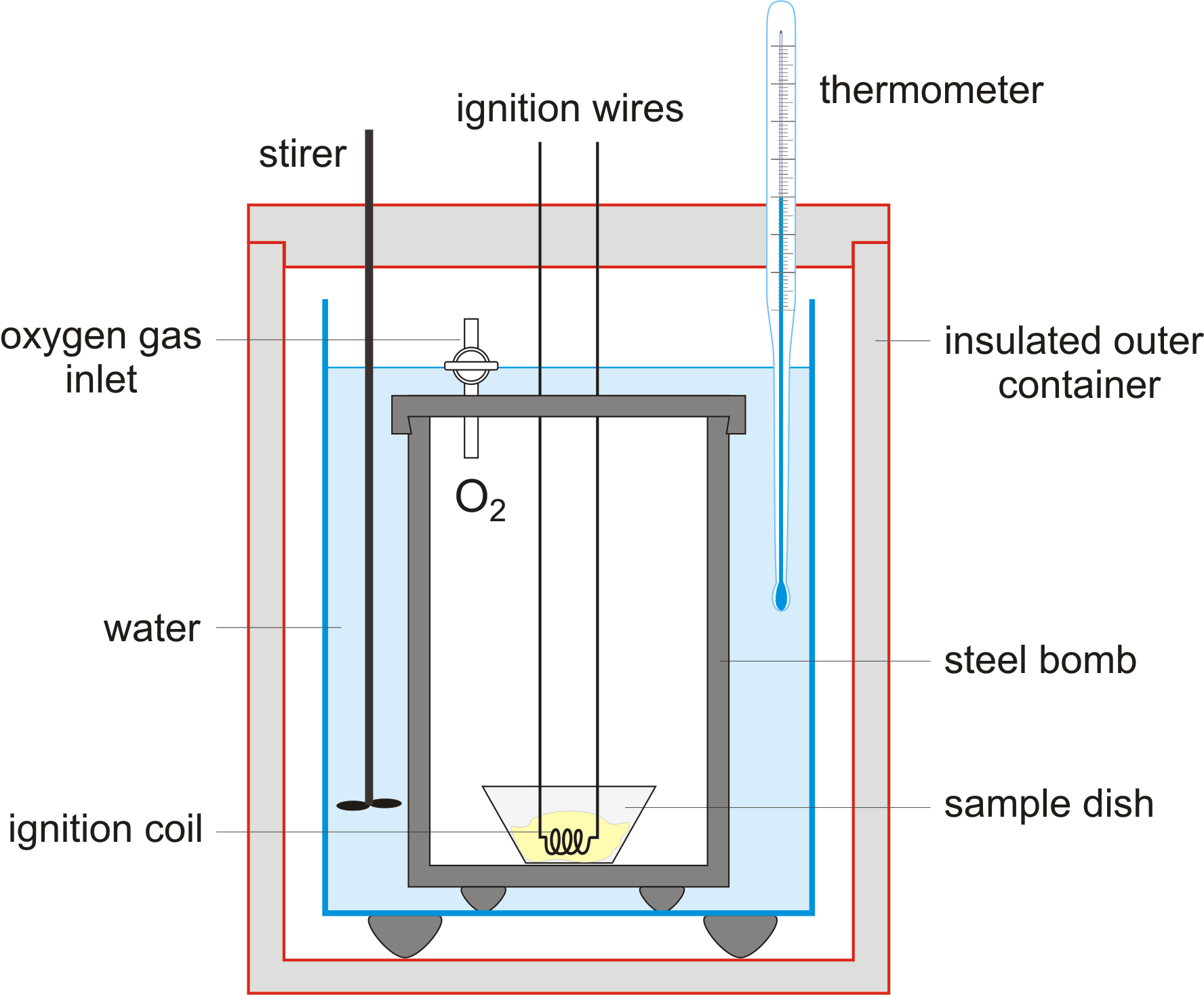
Describe Bomb Calorimeter . a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. four essential parts are required in any bomb calorimeter: definition of bomb calorimeters: the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The consequence of the calculation is called the amount of combustion, calorification, or btu. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. Inside the calorimeter is a vessel in which the. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. A bomb or vessel in which the combustible charges can be burned.
from martinfersbanks.blogspot.com
definition of bomb calorimeters: The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb or vessel in which the combustible charges can be burned. Inside the calorimeter is a vessel in which the. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. four essential parts are required in any bomb calorimeter: the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance.
Is a Bomb Calorimeter Constant Pressure
Describe Bomb Calorimeter the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. four essential parts are required in any bomb calorimeter: Inside the calorimeter is a vessel in which the. a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. definition of bomb calorimeters: A bomb or vessel in which the combustible charges can be burned. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The consequence of the calculation is called the amount of combustion, calorification, or btu. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Describe Bomb Calorimeter definition of bomb calorimeters: The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. bomb calorimeter denotes the heat liberated by the combustion of. Describe Bomb Calorimeter.
From cezqkouv.blob.core.windows.net
Bomb Calorimeter Is Used For Measuring at Sammy Haygood blog Describe Bomb Calorimeter the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The consequence of the calculation is called the amount of combustion, calorification, or btu. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the. Describe Bomb Calorimeter.
From studylib.net
Bomb Calorimeter Describe Bomb Calorimeter a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. definition of bomb calorimeters: The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. four essential. Describe Bomb Calorimeter.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Describe Bomb Calorimeter A bomb or vessel in which the combustible charges can be burned. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Inside the calorimeter is a vessel in which the. the bomb calorimeter consists of a pressurized (30. Describe Bomb Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Describe Bomb Calorimeter Inside the calorimeter is a vessel in which the. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. definition of bomb calorimeters: four essential parts are required in any bomb calorimeter: the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses. Describe Bomb Calorimeter.
From scitechdidactic.com
Bomb Calorimeter Model TH 101 Scitech Didactic UK Describe Bomb Calorimeter Inside the calorimeter is a vessel in which the. four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. definition of bomb calorimeters: a bomb calorimeter is a. Describe Bomb Calorimeter.
From www.britannica.com
Bomb calorimeter measurement device Britannica Describe Bomb Calorimeter a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. a bomb calorimeter is a device that measures the heat given. Describe Bomb Calorimeter.
From www.scribd.com
Bomb Calorimeter Principle,formula procedure.docx Calorimetry Enthalpy Describe Bomb Calorimeter definition of bomb calorimeters: Inside the calorimeter is a vessel in which the. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. four essential parts are required in any bomb calorimeter: The consequence. Describe Bomb Calorimeter.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Describe Bomb Calorimeter the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. definition of bomb calorimeters: a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under. Describe Bomb Calorimeter.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Describe Bomb Calorimeter four essential parts are required in any bomb calorimeter: bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a. Describe Bomb Calorimeter.
From jumpstarterdiscount.blogspot.com
Which Diagram Is A Bomb Calorimeter Wiring Diagram Describe Bomb Calorimeter The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. The consequence of the calculation is called the amount of combustion, calorification, or btu. definition of bomb calorimeters: a bomb calorimeter is used to. Describe Bomb Calorimeter.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Describe Bomb Calorimeter Inside the calorimeter is a vessel in which the. The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. four essential parts are required in any bomb calorimeter: bomb calorimeter denotes the heat liberated by the. Describe Bomb Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Describe Bomb Calorimeter a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. four essential parts are required in any bomb calorimeter: a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. a. Describe Bomb Calorimeter.
From saylordotorg.github.io
Calorimetry Describe Bomb Calorimeter a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. A bomb or vessel in which the combustible charges can be burned. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic. Describe Bomb Calorimeter.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Describe Bomb Calorimeter The consequence of the calculation is called the amount of combustion, calorification, or btu. four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. a bomb calorimeter is a. Describe Bomb Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry Describe Bomb Calorimeter A bomb or vessel in which the combustible charges can be burned. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. definition of bomb calorimeters: a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. The calorific value of solid. Describe Bomb Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Describe Bomb Calorimeter bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. a bomb calorimeter is a scientific instrument designed for. Describe Bomb Calorimeter.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Describe Bomb Calorimeter The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. four essential parts are required in any bomb calorimeter: bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen. Describe Bomb Calorimeter.
From wps.prenhall.com
Media Portfolio Describe Bomb Calorimeter bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. four essential parts are required in any bomb calorimeter: a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water.. Describe Bomb Calorimeter.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Describe Bomb Calorimeter four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. definition of bomb calorimeters: a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen. Describe Bomb Calorimeter.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Describe Bomb Calorimeter definition of bomb calorimeters: a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The calorific value of solid and liquid. Describe Bomb Calorimeter.
From www.slideserve.com
PPT Bomb Calorimeters PowerPoint Presentation, free download ID4499873 Describe Bomb Calorimeter a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb or vessel in which the combustible charges can be burned. a bomb calorimeter. Describe Bomb Calorimeter.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring Describe Bomb Calorimeter a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. The consequence of the calculation is called the amount of combustion, calorification, or btu. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. definition of bomb calorimeters: a bomb. Describe Bomb Calorimeter.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Describe Bomb Calorimeter The consequence of the calculation is called the amount of combustion, calorification, or btu. Inside the calorimeter is a vessel in which the. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned. Describe Bomb Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Describe Bomb Calorimeter The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel. Describe Bomb Calorimeter.
From www.shutterstock.com
Diagram Bomb Calorimeter 스톡 벡터(로열티 프리) 1612623736 Shutterstock Describe Bomb Calorimeter A bomb or vessel in which the combustible charges can be burned. four essential parts are required in any bomb calorimeter: the bomb calorimeter consists of a pressurized (30 bar) oxygen chamber, called “bomb,” which houses the fuel. The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used. Describe Bomb Calorimeter.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Describe Bomb Calorimeter bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. Inside the calorimeter is a vessel in which the. The consequence of the calculation is called the amount of combustion, calorification,. Describe Bomb Calorimeter.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector Describe Bomb Calorimeter four essential parts are required in any bomb calorimeter: definition of bomb calorimeters: a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb). Describe Bomb Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Describe Bomb Calorimeter The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with. Describe Bomb Calorimeter.
From www.alamy.com
Cross section of a typical bomb calorimeter Stock Photo 24063705 Alamy Describe Bomb Calorimeter a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. Inside the calorimeter is a vessel in which the. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. definition. Describe Bomb Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Describe Bomb Calorimeter The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. Inside the calorimeter is a vessel in which. Describe Bomb Calorimeter.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Describe Bomb Calorimeter The consequence of the calculation is called the amount of combustion, calorification, or btu. The calorific value of solid and liquid fuels is determined in the laboratory by ‘bomb calorimeter’ it is so named shape resembles that of a bomb.fig shows the schematic sketch of the bomb calorimeter. a bomb calorimeter is a device that measures the heat given. Describe Bomb Calorimeter.
From brainly.in
Draw the diagram of bomb calorimeter. Brainly.in Describe Bomb Calorimeter a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. definition of bomb calorimeters: bomb calorimeter denotes the heat liberated by the combustion of all carbon and hydrogen with oxygen to form carbon. A bomb or vessel in which the combustible charges can be burned. a bomb calorimeter is. Describe Bomb Calorimeter.
From cider.uoregon.edu
Solution Energetics Heat of Solution Calorimetry Using Demonstrations Describe Bomb Calorimeter a bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. The consequence of the calculation is called the amount of combustion, calorification, or btu. A bomb or vessel in which the combustible charges can be burned. Inside the calorimeter is a vessel in which the. The calorific value of solid and liquid. Describe Bomb Calorimeter.
From byjus.com
What is bomb calorimeter? Describe Bomb Calorimeter a bomb calorimeter is a device that measures the heat given off or taken in by a reaction. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. definition of bomb calorimeters: bomb calorimeter denotes the heat. Describe Bomb Calorimeter.