Magnesium Hydroxide Titration . complexometric titration of magnesium (2.5.11). The low solubility of magnesium hydroxide can be. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. this video explains how to titrate the amount of magnesium. because of its relative simplicity; Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. And a few glass beads,. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is.
from www.numerade.com
complexometric titration of magnesium (2.5.11). Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of magnesium. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. And a few glass beads,. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. because of its relative simplicity; The low solubility of magnesium hydroxide can be. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is.
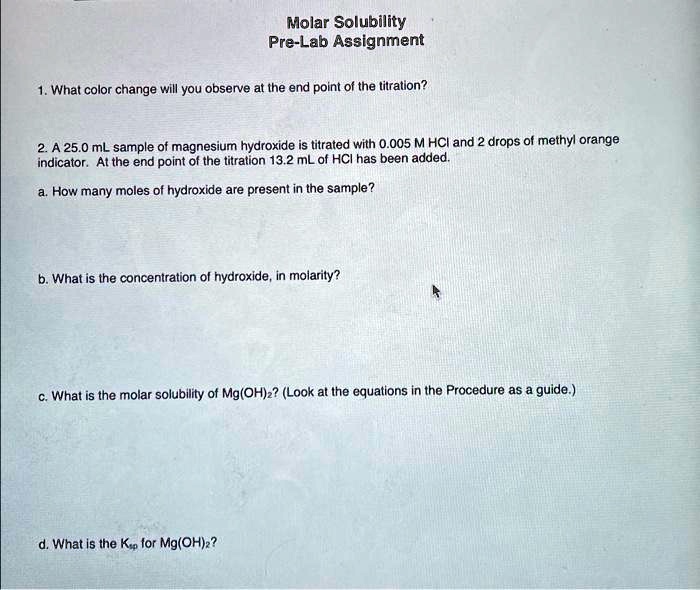
SOLVED Molar Solubility PreLab Assignment 1. What color change will
Magnesium Hydroxide Titration Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. this video explains how to titrate the amount of magnesium. because of its relative simplicity; And a few glass beads,. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. The low solubility of magnesium hydroxide can be. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. complexometric titration of magnesium (2.5.11). Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid.
From mavink.com
Magnesium And Hcl Reaction Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. this video explains how to titrate the amount of magnesium. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid.. Magnesium Hydroxide Titration.
From testbook.com
Magnesium Hydroxide Definition, Symbol, Examples, Structure Magnesium Hydroxide Titration magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. because of its relative simplicity;. Magnesium Hydroxide Titration.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Magnesium Hydroxide Titration magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. And a few glass beads,. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is.. Magnesium Hydroxide Titration.
From www.chegg.com
Solved If 0.607 g of magnesium hydroxide reacts with 0.870 g Magnesium Hydroxide Titration because of its relative simplicity; And a few glass beads,. The low solubility of magnesium hydroxide can be. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. complexometric titration of magnesium (2.5.11). this video explains. Magnesium Hydroxide Titration.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Titration the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. complexometric titration of magnesium (2.5.11). Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. because of its relative simplicity; And a few glass. Magnesium Hydroxide Titration.
From www.researchgate.net
TG and DTG of magnesium hydroxide. Download Scientific Diagram Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. complexometric titration of magnesium (2.5.11). because of its relative simplicity; Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined by the addition of eriochrome black t, which. Magnesium Hydroxide Titration.
From www.chegg.com
Solved 2. Titration Analysis Magnesium hydroxide a strong Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. because of its relative simplicity; Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. the endpoint of the titration is determined by the addition of eriochrome black t, which. Magnesium Hydroxide Titration.
From solvedlib.com
Magnesium hydroxide reacts with hydrochloric acid to … SolvedLib Magnesium Hydroxide Titration Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. complexometric titration of magnesium (2.5.11). Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes. Magnesium Hydroxide Titration.
From prescriptiongiant.com
Magnesium Hydroxide Prescriptiongiant Magnesium Hydroxide Titration Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. complexometric titration of magnesium (2.5.11). Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be.. Magnesium Hydroxide Titration.
From bramblechemistry.weebly.com
4C6 Titration Magnesium Hydroxide Titration complexometric titration of magnesium (2.5.11). this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. magnesium hydroxide is to be dissolved in excess of 1 n. Magnesium Hydroxide Titration.
From www.researchgate.net
Results of thermogravimetric analysis of magnesium hydroxides Magnesium Hydroxide Titration Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. complexometric titration of magnesium (2.5.11). And a few glass beads,. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. Dissolve 1 g of magnesium. Magnesium Hydroxide Titration.
From www.vrogue.co
Titration Colour Changes Slss Science Limerick Educat vrogue.co Magnesium Hydroxide Titration Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. The low solubility of magnesium hydroxide can be. complexometric titration of magnesium (2.5.11). the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the. Magnesium Hydroxide Titration.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Magnesium Hydroxide Titration magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored. Magnesium Hydroxide Titration.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA Magnesium Hydroxide Titration because of its relative simplicity; And a few glass beads,. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of magnesium. . Magnesium Hydroxide Titration.
From exopiharb.blob.core.windows.net
Magnesium Hydroxide And Formula at Mary Starkes blog Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. And a few glass beads,. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. the endpoint of the titration is determined by the. Magnesium Hydroxide Titration.
From www.dreamstime.com
Magnesium Hydroxide Molecular Structure, 3d Model Molecule, Food Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. complexometric titration of magnesium (2.5.11). this video explains how. Magnesium Hydroxide Titration.
From www.slideserve.com
PPT What type of reaction? HCl + NaOH H 2 O + NaCl PowerPoint Magnesium Hydroxide Titration the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. complexometric titration of magnesium (2.5.11). because of its relative simplicity; The low solubility of magnesium hydroxide can be. this video explains how to titrate the amount. Magnesium Hydroxide Titration.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Magnesium Hydroxide Titration complexometric titration of magnesium (2.5.11). The low solubility of magnesium hydroxide can be. this video explains how to titrate the amount of magnesium. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. because of its relative simplicity; the endpoint of the. Magnesium Hydroxide Titration.
From www.researchgate.net
Contact Angle of magnesium hydroxide before and after modification Magnesium Hydroxide Titration this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. And. Magnesium Hydroxide Titration.
From www.numerade.com
SOLVEDFor the dissolution of magnesium hydroxide in water (a) Write a Magnesium Hydroxide Titration Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. complexometric titration of magnesium (2.5.11). The low solubility of magnesium hydroxide can be. this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a. Magnesium Hydroxide Titration.
From www.researchgate.net
(a) Molecular structure diagram of magnesium hydroxide (b) Crystal Magnesium Hydroxide Titration this video explains how to titrate the amount of magnesium. magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a. Magnesium Hydroxide Titration.
From www.numerade.com
SOLVEDMagnesium hydroxide is formed from the reaction of magnesium Magnesium Hydroxide Titration Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. The low solubility of magnesium hydroxide can be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. because of its relative simplicity; complexometric titration of magnesium (2.5.11). magnesium hydroxide is to be dissolved in excess of. Magnesium Hydroxide Titration.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Magnesium Hydroxide Titration And a few glass beads,. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. The low solubility of magnesium hydroxide can be. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. complexometric titration. Magnesium Hydroxide Titration.
From pubs.acs.org
Influence of Operational Strategies for the Recovery of Magnesium Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. because of its relative simplicity; Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. complexometric titration of magnesium (2.5.11). And a few glass beads,. this video explains how to titrate the amount of magnesium. the endpoint of the titration is. Magnesium Hydroxide Titration.
From www.chegg.com
Solved pKsp for magnesium hydroxide is 11.05. To "ballpark" Magnesium Hydroxide Titration complexometric titration of magnesium (2.5.11). the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of. Magnesium Hydroxide Titration.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Magnesium Hydroxide Titration Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of magnesium. complexometric titration of magnesium (2.5.11). because of its relative simplicity; The low solubility of magnesium hydroxide can be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric. Magnesium Hydroxide Titration.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide Titration magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. The low solubility of magnesium. Magnesium Hydroxide Titration.
From www.researchgate.net
DRIFT spectra for magnesium hydroxide, hydrotalcite and hydromagnesite Magnesium Hydroxide Titration because of its relative simplicity; And a few glass beads,. complexometric titration of magnesium (2.5.11). magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined. Magnesium Hydroxide Titration.
From www.garrisonminerals.com
How Magnesium Hydroxide is Made Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. And a few glass beads,. the endpoint of the titration is determined by the addition of eriochrome black t, which forms a colored chelate with mg2+ and undergoes a color change when the mg2+ is. complexometric titration of magnesium (2.5.11). because of its relative simplicity; this video explains. Magnesium Hydroxide Titration.
From www.youtube.com
How to Write the Net Ionic Equation for Mg(OH)2 + HCl = MgCl2 + H2O Magnesium Hydroxide Titration magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. because of its relative simplicity; And a few glass beads,. The low solubility of magnesium hydroxide can be. complexometric titration of magnesium (2.5.11). Magnesium hydroxide is precipitated with sodium hydrox ide in the presence. Magnesium Hydroxide Titration.
From www.youtube.com
How to Balance Mg(OH)2 = MgO + H2O (and Type of Reaction) YouTube Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. complexometric titration of magnesium (2.5.11). magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. because of its relative simplicity; the endpoint. Magnesium Hydroxide Titration.
From achs-prod.acs.org
AtHome Titration Magnesium Hydroxide in Milk of Magnesia Using an Magnesium Hydroxide Titration Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. And a few glass beads,. The low solubility of magnesium hydroxide can be. because of its relative simplicity; this video explains how to titrate the amount of magnesium. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid.. Magnesium Hydroxide Titration.
From www.nagwa.com
Question Video Calculating the Molar Concentration of Mg(OH)₂ Using Magnesium Hydroxide Titration complexometric titration of magnesium (2.5.11). The low solubility of magnesium hydroxide can be. And a few glass beads,. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. the endpoint of the titration is determined by the addition. Magnesium Hydroxide Titration.
From www.numerade.com
SOLVED Molar Solubility PreLab Assignment 1. What color change will Magnesium Hydroxide Titration The low solubility of magnesium hydroxide can be. Dissolve 1 g of magnesium hydroxide to a suitable flask add 20ml of 3 n hydrochloric acid. Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. this video explains how to titrate the amount of magnesium. the endpoint of the titration is determined by the addition. Magnesium Hydroxide Titration.
From www.youtube.com
Magnesium Hydroxide and Hydrochloric Acid Reaction with Universal Magnesium Hydroxide Titration Magnesium hydroxide is precipitated with sodium hydrox ide in the presence of the. The low solubility of magnesium hydroxide can be. because of its relative simplicity; magnesium hydroxide is to be dissolved in excess of 1 n sulphuric acid and the unreacted excess of this acid is to be. Dissolve 1 g of magnesium hydroxide to a suitable. Magnesium Hydroxide Titration.