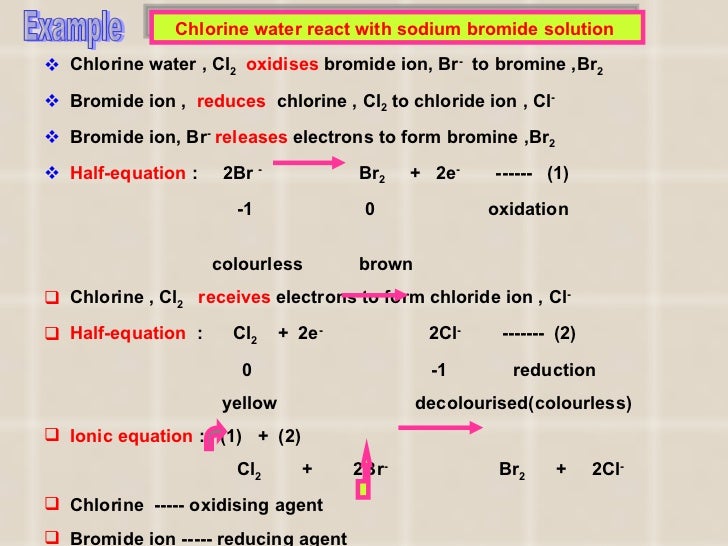
Bromine Water Oxidising Agent . About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Bromine is weaker, and iodine has only mild oxidizing power. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. The reason this reaction is often discussed. Like chlorine water, it is a good. The solution is known as bromine water. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The bromine water test is a simple test to distinguish between glucose and fructose. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o.
from www.slideshare.net
This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The reason this reaction is often discussed. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The bromine water test is a simple test to distinguish between glucose and fructose. Like chlorine water, it is a good. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The solution is known as bromine water. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. Bromine is weaker, and iodine has only mild oxidizing power.
Oxidation & reduction
Bromine Water Oxidising Agent Like chlorine water, it is a good. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. Bromine is weaker, and iodine has only mild oxidizing power. The solution is known as bromine water. Like chlorine water, it is a good. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The bromine water test is a simple test to distinguish between glucose and fructose. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. The reason this reaction is often discussed.
From www.youtube.com
How To Find The Oxidizing and Reducing Agent YouTube Bromine Water Oxidising Agent Like chlorine water, it is a good. The solution is known as bromine water. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): The reason this reaction is often discussed. Bromine is weaker, and iodine has only mild oxidizing power. Aldehydes,. Bromine Water Oxidising Agent.
From merinyjoreenmatias.weebly.com
TASK 2 OXIDATION AND REDUCTION PRINCIPLES IN BIOCHEMISTRY Bromine Water Oxidising Agent The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): Bromine is weaker, and iodine has only mild oxidizing power. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Bromine Water Oxidising Agent The reason this reaction is often discussed. Like chlorine water, it is a good. The solution is known as bromine water. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with.. Bromine Water Oxidising Agent.
From shopwesterntubpoolspa.com
Aqua Brite Plus A NonChlorine/Bromine Oxidizing Agent For Shock Treatment Of Swimming Pools Bromine Water Oxidising Agent The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The bromine water test is a simple test to distinguish between glucose and fructose. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. The solution. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Bromine Water Oxidising Agent The solution is known as bromine water. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. Bromine is weaker, and iodine has only mild oxidizing power. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. This page examines the. Bromine Water Oxidising Agent.
From www.slideshare.net
Oxidation & reduction Bromine Water Oxidising Agent About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The solution is known as bromine water. Bromine is weaker, and iodine has only mild oxidizing power. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. The bromine water test is a simple test to distinguish between glucose. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Water Oxidising Agent The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The reason this reaction is often discussed. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. This page examines the trend in oxidizing ability of the group 17 elements (the. Bromine Water Oxidising Agent.
From chiangmaiplaces.net
Is Bromine Water An Oxidising Agent? Trust The Answer Bromine Water Oxidising Agent About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The reason this reaction is often discussed. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. Aldehydes, including aldoses, are oxidized to their respective carboxylic. Bromine Water Oxidising Agent.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine to bromate and bromide Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. Bromine is weaker, and iodine has only mild oxidizing power. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Like chlorine water, it is a good. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b. Bromine Water Oxidising Agent.
From www.youtube.com
Identifying reducing and oxidizing agents experiment YouTube Bromine Water Oxidising Agent The reason this reaction is often discussed. Like chlorine water, it is a good. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): About 3.41 grams (0.12 ounce) of. Bromine Water Oxidising Agent.
From slidetodoc.com
Oxidizing Reducing Agents Oxidizing Agents An oxidizing agent Bromine Water Oxidising Agent About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): Bromine is weaker, and iodine has only mild oxidizing power. The solution is known as bromine water. Oxygen gas, which constitutes about 20 percent of the earth’s. Bromine Water Oxidising Agent.
From chiangmaiplaces.net
Is Bromine Water An Oxidising Agent? Trust The Answer Bromine Water Oxidising Agent Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The reason this reaction is often discussed. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water. Bromine Water Oxidising Agent.
From www.vedantu.com
Revision Notes Class 11 Chemistry Ch 8 Redox Reactions Bromine Water Oxidising Agent Bromine is weaker, and iodine has only mild oxidizing power. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6961913 Bromine Water Oxidising Agent The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. The bromine water test is a simple test to. Bromine Water Oxidising Agent.
From slideplayer.com
1.5 b Learning apply knowledge of oxidation and reduction to explain the rusting of Bromine Water Oxidising Agent The reason this reaction is often discussed. The bromine water test is a simple test to distinguish between glucose and fructose. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. This page examines the trend in oxidizing ability of the group 17 elements. Bromine Water Oxidising Agent.
From www.alvimcleantech.com
Redox potential Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. This page examines the trend in oxidizing ability of the group 17 elements (the halogens): This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The reason this reaction is. Bromine Water Oxidising Agent.
From slideplayer.com
Chemical oxidation Reactants Products Reduced Oxidized Oxidants ppt download Bromine Water Oxidising Agent The solution is known as bromine water. Like chlorine water, it is a good. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The reason this reaction is often discussed. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical. Bromine Water Oxidising Agent.
From www.slideshare.net
Oxidation & reduction Bromine Water Oxidising Agent The reason this reaction is often discussed. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Like chlorine water, it is a good. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. Bromine is. Bromine Water Oxidising Agent.
From www.chemistrystudent.com
Group 7 Halogens Oxidising Power (ALevel) ChemistryStudent Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. About 3.41 grams (0.12 ounce) of bromine dissolve. Bromine Water Oxidising Agent.
From www.numerade.com
SOLVEDBromine is obtained from sea water by the following reaction Cl2(g)+2 NaBr(aq) →2 NaCl Bromine Water Oxidising Agent This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The reason this reaction is often discussed. Like chlorine water, it is a good. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. This page examines the trend in oxidizing. Bromine Water Oxidising Agent.
From www.researchgate.net
Comparison between various oxidizing agents\KBr systems for the... Download Scientific Diagram Bromine Water Oxidising Agent The solution is known as bromine water. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Like chlorine water, it is a good. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. Bromine is weaker, and iodine has only. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Water Oxidising Agent Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. Bromine is weaker, and iodine has only mild oxidizing power. Like chlorine water, it is a good. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent. Bromine Water Oxidising Agent.
From www.youtube.com
Bromine Water Test Explained// HSC Chemistry YouTube Bromine Water Oxidising Agent About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The bromine water test is a simple test to distinguish between glucose and fructose. This page examines the trend in oxidizing ability of the group 17 elements (the halogens):. Bromine Water Oxidising Agent.
From www.slideshare.net
Oxidation & reduction Bromine Water Oxidising Agent Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Like chlorine water, it is a good. The bromine water test is a simple test to distinguish. Bromine Water Oxidising Agent.
From chem.libretexts.org
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. About 3.41 grams (0.12 ounce) of bromine dissolve. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation, free download ID318185 Bromine Water Oxidising Agent The reason this reaction is often discussed. The bromine water test is a simple test to distinguish between glucose and fructose. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Like chlorine water, it is a good. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2. Bromine Water Oxidising Agent.
From www.chemistore.co.za
Bromine water, 500ml Bromine Water Oxidising Agent The reason this reaction is often discussed. Like chlorine water, it is a good. The bromine water test is a simple test to distinguish between glucose and fructose. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. The glucose undergoes an oxidation reaction to. Bromine Water Oxidising Agent.
From www.youtube.com
Experiment of Bromine Water Test🔥 Organic Chemistry Imran Sir Vibrant Academy YouTube Bromine Water Oxidising Agent This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The solution is. Bromine Water Oxidising Agent.
From chemistrytalk.org
Common Oxidizing Agents & Reducing Agents ChemTalk Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. The solution is known as bromine water.. Bromine Water Oxidising Agent.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Bromine Water Oxidising Agent Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. Bromine is weaker, and iodine has only mild oxidizing power. The reason this reaction is often discussed. Like chlorine water, it is a good. This page discusses what defines an oxidizing or reducing agent, how. Bromine Water Oxidising Agent.
From www.anyrgb.com
Mineral Sanitizer, shock Chlorination, Dichlorine, oxidizing Agent, Bromine, olfaction, chlorine Bromine Water Oxidising Agent The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Bromine is weaker, and iodine has only mild oxidizing power. Like chlorine water, it is a good. The solution is known as bromine water. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x. Bromine Water Oxidising Agent.
From www.slideshare.net
Carbohydrate 1 Bromine Water Oxidising Agent The bromine water test is a simple test to distinguish between glucose and fructose. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. About 3.41 grams (0.12 ounce) of bromine. Bromine Water Oxidising Agent.
From www.expii.com
Oxidizing and Reducing Agents — Definition & Examples Expii Bromine Water Oxidising Agent About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water at room temperature. Like chlorine water, it is a good. The solution is known as bromine water. The glucose undergoes an oxidation reaction to give glucuronic acid in reaction with. Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. The reason. Bromine Water Oxidising Agent.
From spmchemistry.blog.onlinetuition.com.my
Transfer of Electrons from One Point to Another SPM Chemistry Bromine Water Oxidising Agent Bromine is weaker, and iodine has only mild oxidizing power. This page discusses what defines an oxidizing or reducing agent, how to determine an oxidizing and reducing agent in a chemical reaction, and the importance of. The reason this reaction is often discussed. Like chlorine water, it is a good. The solution is known as bromine water. About 3.41 grams. Bromine Water Oxidising Agent.
From slideplayer.com
1.5 b Learning apply knowledge of oxidation and reduction to explain the rusting of Bromine Water Oxidising Agent Oxygen gas, which constitutes about 20 percent of the earth’s atmosphere, is another. Aldehydes, including aldoses, are oxidized to their respective carboxylic acids in the presence of brx2 b r x 2 in hx2o h x 2 o. Like chlorine water, it is a good. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1 quart) of water. Bromine Water Oxidising Agent.