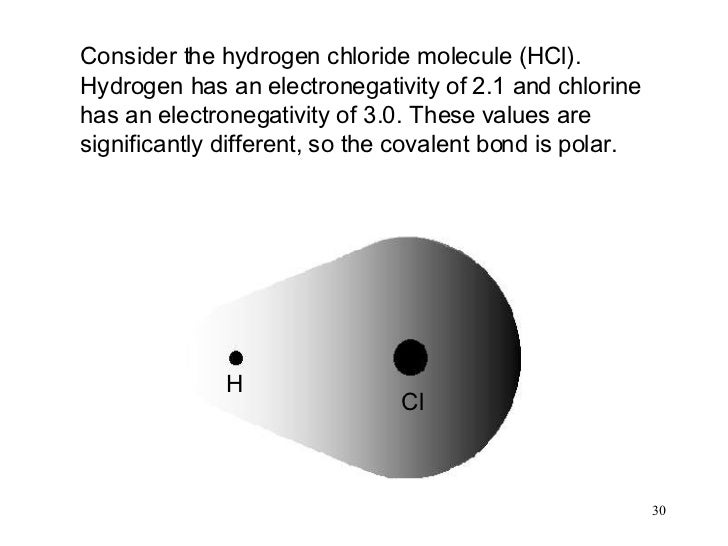
Chlorine Electronegative Than Hydrogen . The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. If b is a lot more electronegative than a, then the electron pair is dragged right. In this guide we’ll break down everything you need to know about electronegativity: In the o 2 molecule,. The pauling scale is the most. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. If you’re studying chemistry, you’ll likely learn about electronegativity. It can also be used to predict if the resulting molecule will be polar. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79;
from www.slideshare.net
It can also be used to predict if the resulting molecule will be polar. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. If you’re studying chemistry, you’ll likely learn about electronegativity. In the o 2 molecule,. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; If b is a lot more electronegative than a, then the electron pair is dragged right. In this guide we’ll break down everything you need to know about electronegativity:
Covalent Bonding Chapter 8
Chlorine Electronegative Than Hydrogen 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. If you’re studying chemistry, you’ll likely learn about electronegativity. If b is a lot more electronegative than a, then the electron pair is dragged right. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In the o 2 molecule,. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; In this guide we’ll break down everything you need to know about electronegativity: The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Chlorine Electronegative Than Hydrogen 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; The pauling scale is the most. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In the o. Chlorine Electronegative Than Hydrogen.
From nl.dreamstime.com
Electronegativity Periodieke Lijst Stock Illustratie Illustratie bestaande uit chemie, linus Chlorine Electronegative Than Hydrogen The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. The pauling scale is the most. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate. Chlorine Electronegative Than Hydrogen.
From pdfslide.net
Electronegativity + 0 0 HClHH The basic units ionic vs. covalent Ionic compounds form Chlorine Electronegative Than Hydrogen A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later. Chlorine Electronegative Than Hydrogen.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Electronegativity Values Chlorine Electronegative Than Hydrogen It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 103 rows the base value of hydrogen was later increased by 0.10. Chlorine Electronegative Than Hydrogen.
From www.alamy.com
Covalent bond of hydrogen chlorine (HCl). Physics resources for teachers and students Stock Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: If b is a lot more electronegative than a, then the electron pair is dragged right. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In the o 2 molecule,. If you’re studying chemistry, you’ll likely learn about electronegativity.. Chlorine Electronegative Than Hydrogen.
From www.slideshare.net
Covalent Bonding Chapter 8 Chlorine Electronegative Than Hydrogen If b is a lot more electronegative than a, then the electron pair is dragged right. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; It can also be used to predict if the resulting molecule will be polar. If you’re studying chemistry, you’ll likely learn about electronegativity. In. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVEDExplain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding Chlorine Electronegative Than Hydrogen A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It can also be used to predict if the resulting molecule will be polar. If b is. Chlorine Electronegative Than Hydrogen.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Chlorine Electronegative Than Hydrogen A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; 119 rows electronegativity is used to predict whether a bond between atoms will be. Chlorine Electronegative Than Hydrogen.
From stock.adobe.com
covalent bond of hydrogen chlorine Stock Vector Adobe Stock Chlorine Electronegative Than Hydrogen 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; In the o 2 molecule,. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this guide we’ll break down everything you need to know about electronegativity: 119 rows electronegativity is used. Chlorine Electronegative Than Hydrogen.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Chlorine Electronegative Than Hydrogen A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. 119 rows electronegativity is used. Chlorine Electronegative Than Hydrogen.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine is 3.6ev/ atom. The Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. The pauling scale is the most. 119 rows electronegativity is used to predict whether a bond between atoms. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVED Given the molecular orbital diagram of the molecule below; we knaw that the Hydrogen Chlorine Electronegative Than Hydrogen In the o 2 molecule,. If you’re studying chemistry, you’ll likely learn about electronegativity. It can also be used to predict if the resulting molecule will be polar. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVED Sodium (Na) and hydrogen (H) have the same number of electrons in their outer shells but Chlorine Electronegative Than Hydrogen The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. If b is a lot more electronegative than a, then the electron pair is dragged right. If you’re studying chemistry, you’ll likely learn about electronegativity. 119 rows electronegativity is used to. Chlorine Electronegative Than Hydrogen.
From www.youtube.com
Covalent Bond of Hydrogen , Chlorine and Hydro Chloride Molecule (Different Kind Of Bonds Chlorine Electronegative Than Hydrogen 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVED In hydrochloric acid (HCI , chlorine has greater electronegativity than hydrogen Chlorine Electronegative Than Hydrogen If you’re studying chemistry, you’ll likely learn about electronegativity. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this guide we’ll break down everything you. Chlorine Electronegative Than Hydrogen.
From www.nagwa.com
Question Video Understanding How Electron Density Is Distributed in Hydrogen Chloride Molecules Chlorine Electronegative Than Hydrogen 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; The pauling scale is the most. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’. Chlorine Electronegative Than Hydrogen.
From www.youtube.com
Electronegativity, Basic Introduction, Periodic Trends Which Element Is More Electronegative Chlorine Electronegative Than Hydrogen 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Chlorine Electronegative Than Hydrogen.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Electronegative Than Hydrogen If b is a lot more electronegative than a, then the electron pair is dragged right. It can also be used to predict if the resulting molecule will be polar. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In the o 2 molecule,. Electronegativity is a measure of the tendency of. Chlorine Electronegative Than Hydrogen.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF] Periodic Table Chlorine Electronegative Than Hydrogen The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. In the o 2 molecule,. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVEDEthene; CH;CH_ has a high electron density around the carboncarbon double bond Hydrogen Chlorine Electronegative Than Hydrogen 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; The pauling scale is the most. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be. Chlorine Electronegative Than Hydrogen.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Britannica Chlorine Electronegative Than Hydrogen If you’re studying chemistry, you’ll likely learn about electronegativity. A chlorine atom is more electronegative than a hydrogen, and thus is able to ‘induce’, or ‘pull’ electron density towards itself, away from the carboxylate group. In the o 2 molecule,. If b is a lot more electronegative than a, then the electron pair is dragged right. It can also be. Chlorine Electronegative Than Hydrogen.
From www.nagwa.com
Question Video Identifying the Statement That Describes How a Single Covalent Bond Is Formed Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The chlorine atom has a higher electronegativity than the hydrogen. Chlorine Electronegative Than Hydrogen.
From www.pinterest.com
Electronegativity Definition and Trend Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: The pauling scale is the most. If you’re studying chemistry, you’ll likely learn about electronegativity. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. If b is a lot more electronegative than a, then the electron pair is dragged. Chlorine Electronegative Than Hydrogen.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Electronegative Than Hydrogen In the o 2 molecule,. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. If you’re studying chemistry, you’ll likely learn about electronegativity. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.. Chlorine Electronegative Than Hydrogen.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine Electronegative Than Hydrogen Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; In this guide we’ll break down everything you need to know about electronegativity: A chlorine atom is more electronegative than a hydrogen, and. Chlorine Electronegative Than Hydrogen.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Electronegativity Values Chlorine Electronegative Than Hydrogen It can also be used to predict if the resulting molecule will be polar. The pauling scale is the most. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. In the o 2 molecule,. If you’re studying chemistry, you’ll likely. Chlorine Electronegative Than Hydrogen.
From slideplayer.com
Chemical Bonding and VSEPR ppt download Chlorine Electronegative Than Hydrogen The pauling scale is the most. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. If you’re studying chemistry, you’ll likely learn about electronegativity. If b is a lot more electronegative than a, then the electron pair is dragged right. In the o 2 molecule,. A chlorine atom is more electronegative than. Chlorine Electronegative Than Hydrogen.
From slideplayer.com
Chemical Bonds The Formation of Compounds From Atoms ppt download Chlorine Electronegative Than Hydrogen The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. A chlorine atom is more electronegative than a hydrogen, and thus is able to. Chlorine Electronegative Than Hydrogen.
From material-properties.org
Chlorine and Iodine Comparison Properties Material Properties Chlorine Electronegative Than Hydrogen Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It can also be used to predict if the resulting molecule will be polar. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than to the h in the hcl molecule.. Chlorine Electronegative Than Hydrogen.
From www.numerade.com
SOLVED Given the molecular orbital diagram of the molecule below; we knaw that the Hydrogen Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: It can also be used to predict if the resulting molecule will be polar. In the o 2 molecule,. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; Electronegativity is a measure of the tendency of. Chlorine Electronegative Than Hydrogen.
From ircamera.as.arizona.edu
Spectroscopy Chlorine Electronegative Than Hydrogen 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this guide we’ll break down everything you need to know about electronegativity: It can also be used to predict if the resulting molecule will be. Chlorine Electronegative Than Hydrogen.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Electronegative Than Hydrogen Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In the o 2 molecule,. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl. Chlorine Electronegative Than Hydrogen.
From www.reddit.com
Chem why does chlorine donate electron density to N here? I get that N and Cl are very close in Chlorine Electronegative Than Hydrogen In this guide we’ll break down everything you need to know about electronegativity: 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The pauling scale is the most. In the o 2. Chlorine Electronegative Than Hydrogen.
From www.chemtopper.com
Video quiz on electronegativity part 4 on polarity in organic molecules due to change in Chlorine Electronegative Than Hydrogen 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. In the o 2 molecule,. If b is a lot more electronegative than a, then the electron pair is dragged right. The chlorine atom has a higher electronegativity than the hydrogen atom, so the bonding electrons will be closer to the cl than. Chlorine Electronegative Than Hydrogen.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Electronegative Than Hydrogen The pauling scale is the most. 103 rows the base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79; Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. In this guide we’ll break down everything you need to know about electronegativity: 119 rows electronegativity is. Chlorine Electronegative Than Hydrogen.