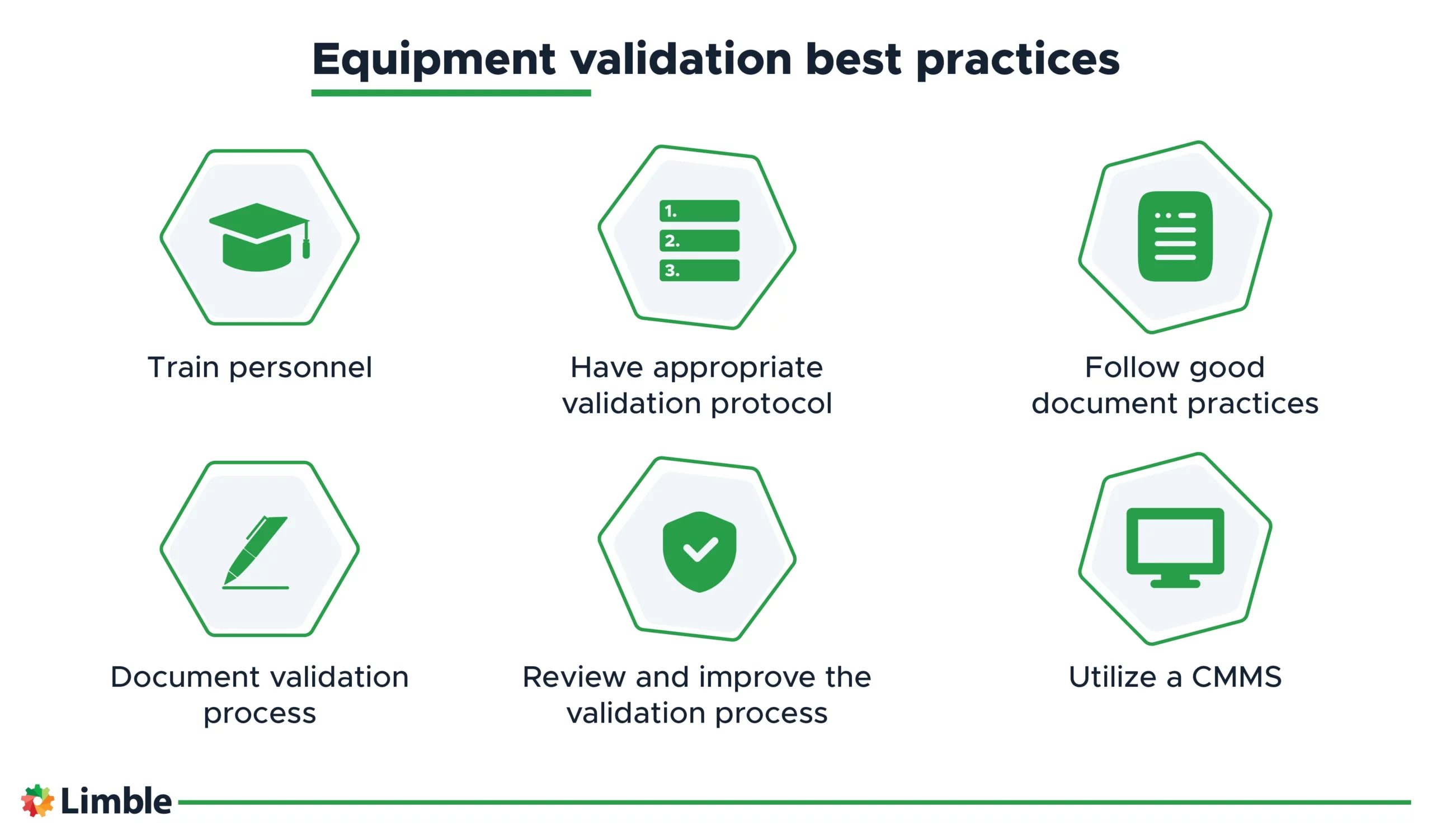
Equipment Validation Examples . One of the key sets of protocols within equipment validation is. The plan should also include a timeline, the responsibilities of team. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. Iq/oq/pq for laboratory equipment validation. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The aim of this validation guideline is to. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Identify critical equipment parameters, acceptance criteria, and validation protocols.
from limblecmms.com
Iq/oq/pq for laboratory equipment validation. The plan should also include a timeline, the responsibilities of team. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. Identify critical equipment parameters, acceptance criteria, and validation protocols. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. One of the key sets of protocols within equipment validation is. The aim of this validation guideline is to.
What is Equipment Validation? Limble CMMS
Equipment Validation Examples One of the key sets of protocols within equipment validation is. The plan should also include a timeline, the responsibilities of team. Iq/oq/pq for laboratory equipment validation. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. One of the key sets of protocols within equipment validation is. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. The aim of this validation guideline is to. Identify critical equipment parameters, acceptance criteria, and validation protocols. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. The process of insuring equipment or system are properly installed or properly operating or properly performing a process.
From www.slideshare.net
EQUIPMENT VALIDATION Equipment Validation Examples Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. One of the key sets of protocols within equipment validation is. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system. Equipment Validation Examples.
From www.presentationeze.com
Equipment Validation Facility Qualification Material QualificationPresentationEZE Equipment Validation Examples Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Iq/oq/pq for. Equipment Validation Examples.
From templates.rjuuc.edu.np
Equipment Validation Protocol Template Equipment Validation Examples This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. The aim of this validation guideline is to. One of the. Equipment Validation Examples.
From limblecmms.com
What is Equipment Validation? Limble CMMS Equipment Validation Examples This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Equipment validation is a systematic and documented. Equipment Validation Examples.
From www.template.net
Validation Report Templates 9+ Free Word, PDF Format Download Equipment Validation Examples The aim of this validation guideline is to. One of the key sets of protocols within equipment validation is. Identify critical equipment parameters, acceptance criteria, and validation protocols. Iq/oq/pq for laboratory equipment validation. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various. Equipment Validation Examples.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd issuu Equipment Validation Examples One of the key sets of protocols within equipment validation is. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. This validation guideline describes the approach and methods which will be used for the qualification of equipment at. Equipment Validation Examples.
From www.slideshare.net
Validation of equipment copy Equipment Validation Examples Iq/oq/pq for laboratory equipment validation. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. The plan should also include a timeline, the responsibilities of team.. Equipment Validation Examples.
From www.getreskilled.com
What's a Pharmaceutical Equipment Validation Protocol & Why is it Crucial? Equipment Validation Examples Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Identify critical equipment parameters, acceptance criteria, and validation protocols. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. An equipment validation protocol (also known. Equipment Validation Examples.
From www.getreskilled.com
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma? Equipment Validation Examples The plan should also include a timeline, the responsibilities of team. The aim of this validation guideline is to. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs. Equipment Validation Examples.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Equipment Validation Examples One of the key sets of protocols within equipment validation is. The aim of this validation guideline is to. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The. Equipment Validation Examples.
From www.presentationeze.com
Equipment Validation FAT, DQ, IQ, OQ, PQ, URS.PresentationEZE Equipment Validation Examples This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and. Equipment Validation Examples.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Equipment Validation Examples Identify critical equipment parameters, acceptance criteria, and validation protocols. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and. Equipment Validation Examples.
From www.slideshare.net
Validation of equipment copy Equipment Validation Examples One of the key sets of protocols within equipment validation is. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. The aim of this validation guideline is to. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. Iq/oq/pq is one way for. Equipment Validation Examples.
From www.slideshare.net
Equipment validation Equipment Validation Examples This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The aim of this validation guideline is to. The plan should also include a timeline, the responsibilities of team. Identify critical equipment parameters, acceptance criteria, and validation protocols. An equipment validation protocol (also known as an equipment qualification. Equipment Validation Examples.
From www.slideshare.net
Equipment validation Equipment Validation Examples Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Iq/oq/pq for laboratory equipment validation. The plan should also include a timeline, the responsibilities of. Equipment Validation Examples.
From www.inpaspages.com
Equipment Validation Report Equipment Validation Examples This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it. Equipment Validation Examples.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Equipment Validation Examples Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. The process of insuring equipment or system are properly installed or properly operating or properly. Equipment Validation Examples.
From limblecmms.com
What is Equipment Validation? Limble CMMS Equipment Validation Examples The process of insuring equipment or system are properly installed or properly operating or properly performing a process. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. Equipment validation. Equipment Validation Examples.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Equipment Validation Examples Iq/oq/pq for laboratory equipment validation. Identify critical equipment parameters, acceptance criteria, and validation protocols. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. Iq/oq/pq is. Equipment Validation Examples.
From www.presentationeze.com
test equipment validation PresentationEZE Equipment Validation Examples The aim of this validation guideline is to. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The plan should also include a timeline, the responsibilities of team. Equipment validation is a systematic and documented process that verifies. Equipment Validation Examples.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Equipment Validation Examples Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. An equipment validation protocol (also known as an equipment qualification protocol). Equipment Validation Examples.
From www.sampletemplates.com
Sample Validation Plan Template 9+ Free Documents in PDF, Word Equipment Validation Examples One of the key sets of protocols within equipment validation is. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The plan should also include a timeline, the responsibilities of team. Identify critical equipment parameters, acceptance criteria, and. Equipment Validation Examples.
From issuu.com
Vt tmp1200 10 validation plan equipment qualification template r02 by PharmOut Issuu Equipment Validation Examples Equipment validation is a systematic and documented process that verifies that equipment, such as machinery, instruments, or software,. The aim of this validation guideline is to. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Iq/oq/pq for laboratory equipment validation. Identify critical. Equipment Validation Examples.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Equipment Validation Examples Identify critical equipment parameters, acceptance criteria, and validation protocols. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. The aim of this validation guideline is to. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well. Equipment Validation Examples.
From www.researchgate.net
Process Validation, Equipment Selection and Qualification Download Scientific Diagram Equipment Validation Examples Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The aim of this validation guideline is to. One of the. Equipment Validation Examples.
From www.validation-online.net
Pharmaceutical Equipment Validation Equipment Validation Examples One of the key sets of protocols within equipment validation is. An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. Iq/oq/pq is one way for. Equipment Validation Examples.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Equipment Validation Examples The plan should also include a timeline, the responsibilities of team. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. One of the key sets of protocols within equipment validation is. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of. Equipment Validation Examples.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Equipment Validation Examples The process of insuring equipment or system are properly installed or properly operating or properly performing a process. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. The aim of this validation guideline is to. Iq/oq/pq for laboratory equipment validation. Equipment validation. Equipment Validation Examples.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Equipment Validation Examples An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of. Equipment Validation Examples.
From www.presentationeze.com
Equipment Validation PresentationEZE Equipment Validation Examples An equipment validation protocol (also known as an equipment qualification protocol) is a written plan that outlines how to test and verify equipment, piping, instruments and utilities in a critical system to make sure it works properly and consistently according to its intended use. The aim of this validation guideline is to. Equipment validation is a systematic and documented process. Equipment Validation Examples.
From www.researchgate.net
Process of equipment validation Download Scientific Diagram Equipment Validation Examples The aim of this validation guideline is to. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Identify critical equipment parameters, acceptance criteria, and. Equipment Validation Examples.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Equipment Validation Examples One of the key sets of protocols within equipment validation is. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The plan should also include a timeline, the responsibilities of team. The aim of this validation guideline is. Equipment Validation Examples.
From www.slideshare.net
Validation of equipments Equipment Validation Examples Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. The process. Equipment Validation Examples.
From www.slideshare.net
Equipment validation Equipment Validation Examples Iq/oq/pq for laboratory equipment validation. The process of insuring equipment or system are properly installed or properly operating or properly performing a process. This validation guideline describes the approach and methods which will be used for the qualification of equipment at a gmp manufacturing site. The aim of this validation guideline is to. Iq/oq/pq is one way for laboratories to. Equipment Validation Examples.
From www.scribd.com
Validation of Equipment Verification And Validation Specification (Technical Standard) Equipment Validation Examples Equipment validation is the process of validating the requirements, specifications, and uses of a piece of equipment to ensure it meets user needs as well as various regulatory and safety requirements. Iq/oq/pq is one way for laboratories to document objective evidence that equipment or instruments are installed correctly, operate effectively and provide valid results under normal operating conditions. An equipment. Equipment Validation Examples.