What Does The Standard Enthalpy Of Formation Tell You . The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their.
from studylib.net
The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen.
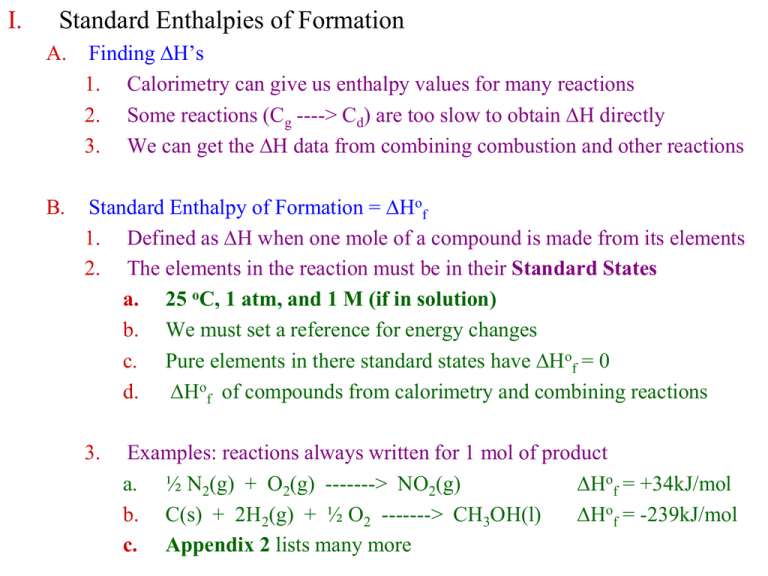
I. Standard Enthalpies of Formation
What Does The Standard Enthalpy Of Formation Tell You 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds.
From study.com
How to Draw & Label Enthalpy Diagrams Lesson What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation for elements in their most stable form. What Does The Standard Enthalpy Of Formation Tell You.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. What Does The Standard Enthalpy Of Formation Tell You.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ),. What Does The Standard Enthalpy Of Formation Tell You.
From mungfali.com
Enthalpy Change Of Formation Equation What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation is a measure of the energy released or consumed. What Does The Standard Enthalpy Of Formation Tell You.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies of Formation YouTube What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation of any element in its standard state is zero by definition.. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideshare.net
Standard enthalpy of formation What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A. What Does The Standard Enthalpy Of Formation Tell You.
From pdfprof.com
enthalpies standard de formation et entropie standard What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A. What Does The Standard Enthalpy Of Formation Tell You.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Change YouTube What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed. What Does The Standard Enthalpy Of Formation Tell You.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube What Does The Standard Enthalpy Of Formation Tell You 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which. What Does The Standard Enthalpy Of Formation Tell You.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Chemistry 2e 5.3 YouTube What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. What Does The Standard Enthalpy Of Formation Tell You.
From studylib.net
Standard Enthalpy of Formation and Reaction What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A standard enthalpy. What Does The Standard Enthalpy Of Formation Tell You.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. 193. What Does The Standard Enthalpy Of Formation Tell You.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine the enthalpy of reaction What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can exist as ozone (o 3. What Does The Standard Enthalpy Of Formation Tell You.
From mungfali.com
Standard Molar Enthalpy Of Formation Table What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free download ID4820880 What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for elements in their most stable. What Does The Standard Enthalpy Of Formation Tell You.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. For. What Does The Standard Enthalpy Of Formation Tell You.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an. What Does The Standard Enthalpy Of Formation Tell You.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation for elements. What Does The Standard Enthalpy Of Formation Tell You.
From studylib.net
I. Standard Enthalpies of Formation What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations. What Does The Standard Enthalpy Of Formation Tell You.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID4097208 What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The. What Does The Standard Enthalpy Of Formation Tell You.
From www.chemistryspace.com
Standard Enthalpy of Formation What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. The standard enthalpy of formation is defined as the change in. What Does The Standard Enthalpy Of Formation Tell You.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps What Does The Standard Enthalpy Of Formation Tell You 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation for elements in their most stable. What Does The Standard Enthalpy Of Formation Tell You.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic. What Does The Standard Enthalpy Of Formation Tell You.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib What Does The Standard Enthalpy Of Formation Tell You 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed. What Does The Standard Enthalpy Of Formation Tell You.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all answers to three sig figs What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A. What Does The Standard Enthalpy Of Formation Tell You.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free download ID228912 What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ),. What Does The Standard Enthalpy Of Formation Tell You.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction What Does The Standard Enthalpy Of Formation Tell You A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from. What Does The Standard Enthalpy Of Formation Tell You.
From www.chegg.com
Solved Use a standard enthalpies of formation table to What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is defined as the change in enthalpy when. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation for elements in their most stable form is defined as zero, simplifying calculations involving compounds.. What Does The Standard Enthalpy Of Formation Tell You.
From mungfali.com
Standard Enthalpy Change Equation What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.. What Does The Standard Enthalpy Of Formation Tell You.
From www.numerade.com
SOLVEDUse average bond energies together with the standard enthalpy of formation of C(g)(718.4 What Does The Standard Enthalpy Of Formation Tell You The standard enthalpy of formation is defined as the change in enthalpy when one mole of a compound is formed from its elements in their. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a. What Does The Standard Enthalpy Of Formation Tell You.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID6642303 What Does The Standard Enthalpy Of Formation Tell You For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed. What Does The Standard Enthalpy Of Formation Tell You.