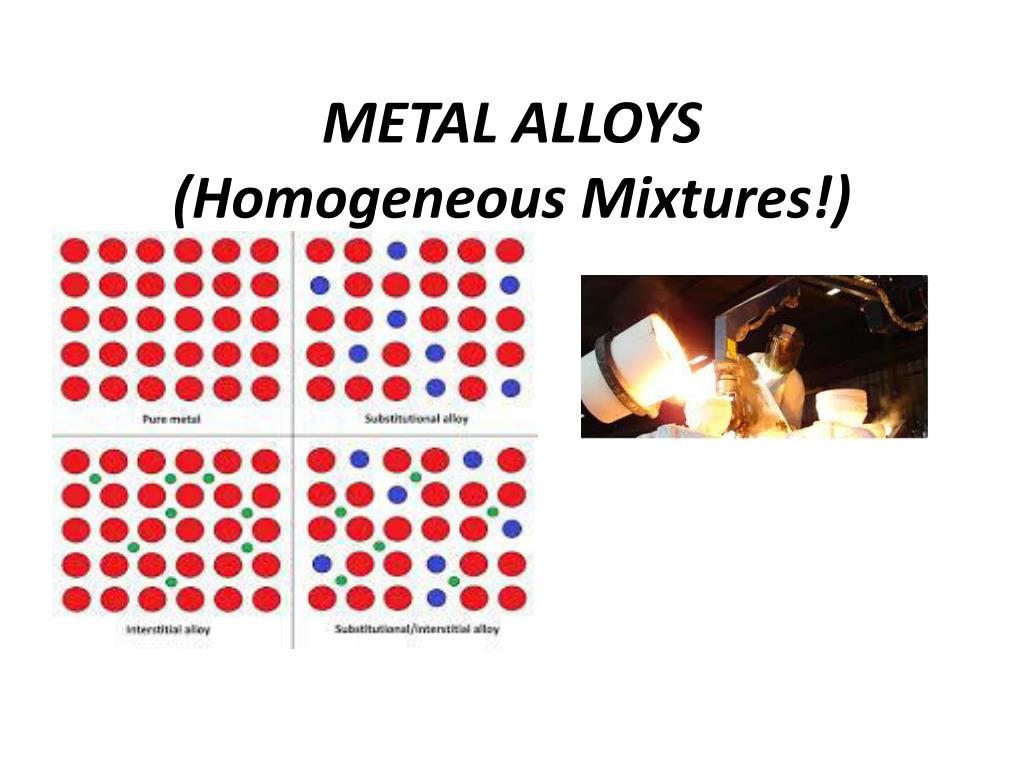
Why Is Stainless Steel A Homogeneous Mixture . A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A heterogeneous mixture consists of two or more. Air is primarily a mixture of. On the other hand, a heterogeneous.
from www.slideserve.com
On the other hand, a heterogeneous. A heterogeneous mixture consists of two or more. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. Air is primarily a mixture of. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. Both steel and stainless steels, which includes chromium, are homogeneous mixtures.
PPT METAL ALLOYS (Homogeneous Mixtures!) PowerPoint Presentation, free download ID2623682
Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A heterogeneous mixture consists of two or more. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Air is primarily a mixture of. On the other hand, a heterogeneous.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID3016356 Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. Air is primarily a mixture of. A homogeneous mixture is combination of two or. Why Is Stainless Steel A Homogeneous Mixture.
From www.slideserve.com
PPT METAL ALLOYS (Homogeneous Mixtures!) PowerPoint Presentation, free download ID2623682 Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. On the other hand, a heterogeneous. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. By definition, a pure substance or a. Why Is Stainless Steel A Homogeneous Mixture.
From www.slideserve.com
PPT CHAPTER 4 MATERIALS METALS AND NON METALS PowerPoint Presentation ID466586 Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. Air is primarily a mixture. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Review. ppt download Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. Air is primarily a mixture of. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Physical Science Period Table—Day 13 Materials Needed Writing Utensil ppt download Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the components that make up the mixture are. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Intro screen. ppt download Why Is Stainless Steel A Homogeneous Mixture A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A heterogeneous mixture consists of two or more. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Both steel and stainless steels, which includes chromium, are. Why Is Stainless Steel A Homogeneous Mixture.
From www.huaxiao-stainless-steel.com
Is Stainless Steel A Homogeneous Mixture? Huaxiao Stainless Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. Air is primarily a mixture of. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. By definition, a pure substance or a homogeneous mixture consists of a single phase. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Substances, Mixtures and Elements ppt download Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. A heterogeneous mixture consists of two or more. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. Air is primarily a mixture of. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed. Why Is Stainless Steel A Homogeneous Mixture.
From www.huaxiao-stainless-steel.com
Is Stainless Steel A Homogeneous Mixture? Huaxiao Stainless Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. By definition, a pure substance or a homogeneous mixture consists of a single phase. Air is primarily a mixture of. A homogeneous mixture is a mixture in. Why Is Stainless Steel A Homogeneous Mixture.
From blog.thepipingmart.com
Is Brass Homogeneous or Heterogeneous? Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A heterogeneous mixture consists of two or more. By definition, a pure substance or a homogeneous mixture consists of a single phase. On the other hand, a heterogeneous.. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Mixtures Matter Elements Compounds Pure Substances Homogenous Mixture ppt download Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is combination of two or more substances that are so intimately. Why Is Stainless Steel A Homogeneous Mixture.
From www.thoughtco.com
10 Heterogeneous and Homogeneous Mixtures Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. Air is primarily a mixture of. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A homogeneous mixture is a mixture in which the composition is uniform throughout. Why Is Stainless Steel A Homogeneous Mixture.
From www.huaxiao-stainless-steel.com
Is Stainless Steel A Homogeneous Mixture? Huaxiao Stainless Why Is Stainless Steel A Homogeneous Mixture By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A heterogeneous mixture consists of two or more. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. Air is primarily a mixture of. A homogeneous mixture is a mixture. Why Is Stainless Steel A Homogeneous Mixture.
From www.slideserve.com
PPT C1 Iron , steel and aluminium PowerPoint Presentation, free download ID2247482 Why Is Stainless Steel A Homogeneous Mixture A heterogeneous mixture consists of two or more. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. Air is primarily a mixture of. By definition, a pure substance or a homogeneous mixture consists of a single phase. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A mixture. Why Is Stainless Steel A Homogeneous Mixture.
From www.slideserve.com
PPT METAL ALLOYS (Homogeneous Mixtures!) PowerPoint Presentation, free download ID2623682 Why Is Stainless Steel A Homogeneous Mixture Air is primarily a mixture of. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A heterogeneous mixture consists of two or more. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A homogeneous mixture is a mixture. Why Is Stainless Steel A Homogeneous Mixture.
From sciencetrends.com
5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A mixture of iron, carbon, and other elements, stainless. Why Is Stainless Steel A Homogeneous Mixture.
From blog.thepipingmart.com
Stainless Steel vs Black Stainless Steel What's the Difference Why Is Stainless Steel A Homogeneous Mixture A heterogeneous mixture consists of two or more. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A homogeneous mixture is a mixture in which the composition is. Why Is Stainless Steel A Homogeneous Mixture.
From sciencenotes.org
What Is a Homogeneous Mixture? Definition and Examples Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. On the other hand, a heterogeneous. Air is primarily a mixture of. A heterogeneous mixture consists of two or more. A mixture of iron, carbon, and other elements,. Why Is Stainless Steel A Homogeneous Mixture.
From www.stfuandplay.com
What do you need to know about heterogeneous and homogeneous mixtures? Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A heterogeneous mixture consists of two or more. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Elements, Compounds & Mixtures! Mr. Coffey. ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Air is primarily a mixture of. On the other hand, a heterogeneous. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. Both steel and stainless steels, which includes chromium,. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Mixtures. ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the components that make. Why Is Stainless Steel A Homogeneous Mixture.
From www.teachoo.com
Class 10 Exemplar MCQ Alloys are homogeneous mixtures of a metal Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. On the other hand, a heterogeneous. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. A heterogeneous mixture consists of two or more. By definition, a pure substance or. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Matter & Change Chapter 1 Sections 1 & 2 ppt download Why Is Stainless Steel A Homogeneous Mixture On the other hand, a heterogeneous. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. Air is primarily a mixture of. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. A homogeneous mixture is a mixture in. Why Is Stainless Steel A Homogeneous Mixture.
From www.huaxiao-stainless-steel.com
Is Stainless Steel A Homogeneous Mixture? Huaxiao Stainless Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is combination of two or more substances that are so. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Chapter 3 Earth’s Environmental Systems ppt download Why Is Stainless Steel A Homogeneous Mixture Air is primarily a mixture of. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A heterogeneous mixture consists of two or more. A. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
The Science of Matter Chapter 1 ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. On the other hand, a heterogeneous. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. Air is primarily a mixture of. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Mixtures. ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. Air is primarily a mixture of. A homogeneous mixture is a mixture in which the components that make up. Why Is Stainless Steel A Homogeneous Mixture.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A heterogeneous mixture consists of two or more. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Chapter 2 Energy and Matter ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A heterogeneous mixture consists of two or more. Air is primarily a mixture of. On the other hand, a heterogeneous. A homogeneous mixture is combination of two or. Why Is Stainless Steel A Homogeneous Mixture.
From 88guru.com
What Are Homogeneous Mixtures Classification and Applications 88Guru Why Is Stainless Steel A Homogeneous Mixture A heterogeneous mixture consists of two or more. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. Both steel and stainless steels, which includes chromium, are homogeneous mixtures. Air is primarily a. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Chemistry is the study of… ppt download Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. By definition, a pure substance or a homogeneous mixture consists of a single phase. On the other hand, a heterogeneous. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A homogeneous mixture is a mixture. Why Is Stainless Steel A Homogeneous Mixture.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. Air is primarily a mixture of. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components.. Why Is Stainless Steel A Homogeneous Mixture.
From slideplayer.com
Bellwork Chapter Pretest ppt download Why Is Stainless Steel A Homogeneous Mixture Both steel and stainless steels, which includes chromium, are homogeneous mixtures. Air is primarily a mixture of. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. By definition, a pure substance or. Why Is Stainless Steel A Homogeneous Mixture.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID9660645 Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A mixture of iron, carbon, and other elements, stainless steel is a homogeneous material due to the even distribution of its components. By definition, a pure substance or a homogeneous mixture consists of a single phase. A homogeneous mixture is a. Why Is Stainless Steel A Homogeneous Mixture.
From pdfprof.com
steel is homogeneous or heterogeneous mixture Why Is Stainless Steel A Homogeneous Mixture A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. On the other hand, a heterogeneous. A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves. A heterogeneous mixture consists of two or more. By definition, a pure substance or. Why Is Stainless Steel A Homogeneous Mixture.