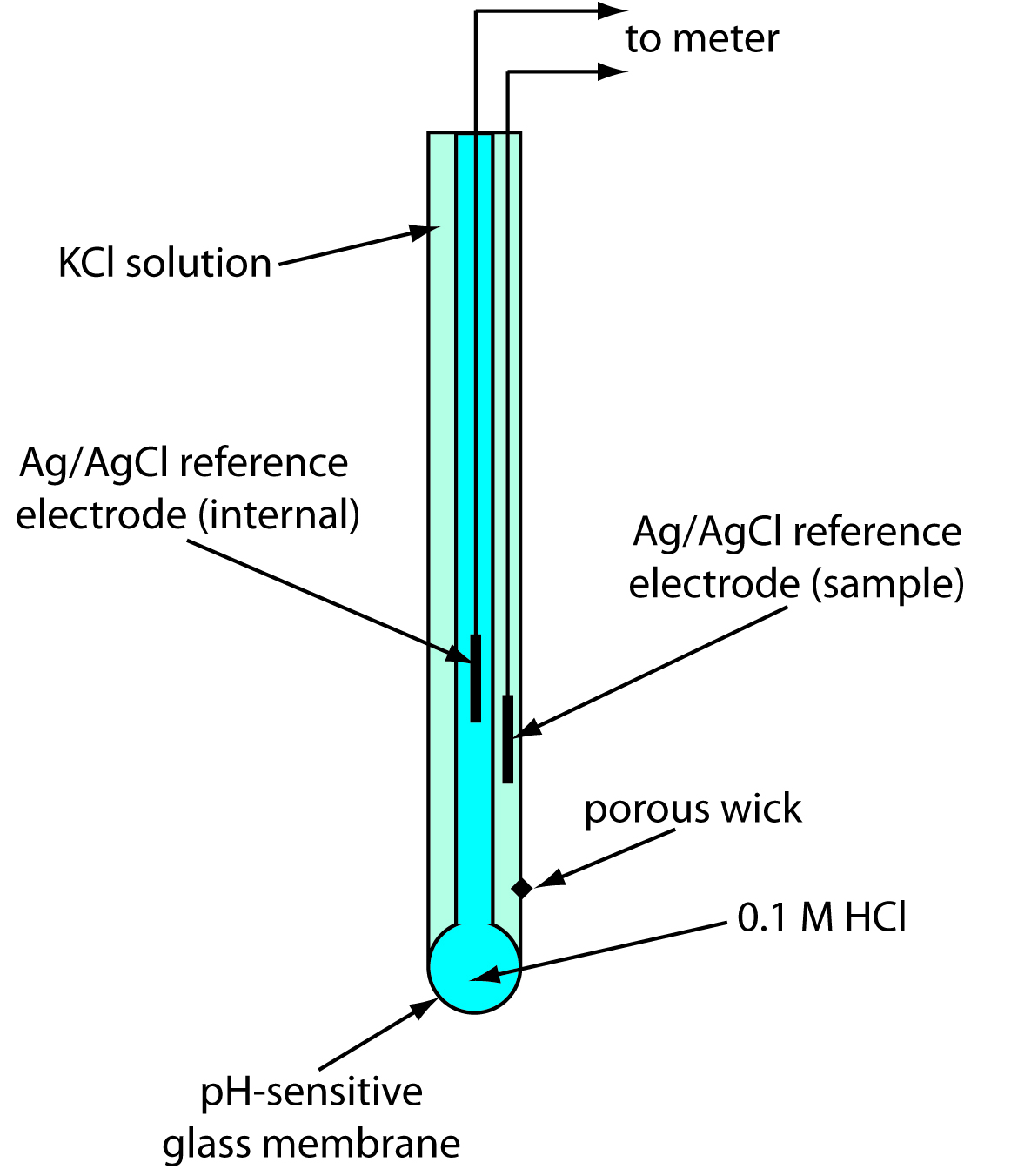
Explain Electrode In Chemistry . electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. That’s why scientists refer to metals as conductors: electrodes are vital components of electrochemical cells. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is called electricity, which. — metals offer a great path. electrochemistry is the study of chemical processes that cause electrons to move. A semiconductor, an electrolyte, a vacuum or. But to move electricity through non. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g.
from chem.libretexts.org
electrodes are vital components of electrochemical cells. That’s why scientists refer to metals as conductors: electrochemical cells allow measurement and control of a redox reaction. electrochemistry is the study of chemical processes that cause electrons to move. The reaction can be started and stopped by connecting or disconnecting the two electrodes. A semiconductor, an electrolyte, a vacuum or. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. — metals offer a great path. But to move electricity through non. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob.
11.2 Potentiometric Methods Chemistry LibreTexts
Explain Electrode In Chemistry electrochemical cells allow measurement and control of a redox reaction. A semiconductor, an electrolyte, a vacuum or. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. This movement of electrons is called electricity, which. But to move electricity through non. electrochemistry is the study of chemical processes that cause electrons to move. The reaction can be started and stopped by connecting or disconnecting the two electrodes. electrodes are vital components of electrochemical cells. — metals offer a great path. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. electrochemical cells allow measurement and control of a redox reaction. That’s why scientists refer to metals as conductors:
From 2012books.lardbucket.org
Electrochemistry Explain Electrode In Chemistry — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. electrodes are vital components of electrochemical cells. This movement of electrons is called electricity,. Explain Electrode In Chemistry.
From brainly.in
Standard hydrogen electrode explain Brainly.in Explain Electrode In Chemistry But to move electricity through non. — metals offer a great path. A semiconductor, an electrolyte, a vacuum or. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. electrochemistry is the study of chemical processes that cause electrons to move. If we place a variable resistance in the. Explain Electrode In Chemistry.
From studylib.net
electrochemistry 2 Explain Electrode In Chemistry — metals offer a great path. electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. electrodes are vital components of electrochemical. Explain Electrode In Chemistry.
From www.schuettemetals.com
The Marvelous Alchemy of Electroplating Explain Electrode In Chemistry But to move electricity through non. This movement of electrons is called electricity, which. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A semiconductor,. Explain Electrode In Chemistry.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Explain Electrode In Chemistry electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which. That’s why scientists refer to metals as conductors: — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. an electrode is an electrical conductor used to make contact. Explain Electrode In Chemistry.
From www.youtube.com
Chapter 15 Solid State Electrodes CHM 214 146 YouTube Explain Electrode In Chemistry electrodes are vital components of electrochemical cells. That’s why scientists refer to metals as conductors: electrochemical cells allow measurement and control of a redox reaction. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. an electrode is an electrical conductor used to make contact with a. Explain Electrode In Chemistry.
From exoiwzsuc.blob.core.windows.net
What Are The Different Types Of Electrodes at Elenora Spink blog Explain Electrode In Chemistry electrochemistry is the study of chemical processes that cause electrons to move. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. electrochemical cells allow measurement and control of a redox reaction. That’s why scientists refer to metals as conductors: — an. Explain Electrode In Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Explain Electrode In Chemistry an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. The reaction can be started and stopped by connecting or disconnecting the two electrodes. electrochemistry is the study of chemical processes that cause electrons to move. That’s why scientists refer to metals as conductors: — metals offer a great. Explain Electrode In Chemistry.
From byjus.com
What is an active electrode? Explain with the help of an example Explain Electrode In Chemistry an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A semiconductor, an electrolyte, a vacuum or. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This movement of electrons is called electricity, which. electrochemical cells allow measurement and. Explain Electrode In Chemistry.
From mavink.com
Electrode Classification Diagram Explain Electrode In Chemistry electrodes are vital components of electrochemical cells. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. The reaction can be started and stopped. Explain Electrode In Chemistry.
From icsechemistry16.blogspot.com
Electrolysis of copper sulphate using copper electrodes Explain Electrode In Chemistry The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. — metals offer a great path. electrochemistry is the study of chemical processes that cause electrons to move. . Explain Electrode In Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Explain Electrode In Chemistry If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. electrochemical cells allow measurement and control of a redox reaction. — metals offer a great path. electrochemistry is the study of chemical processes that cause electrons to move. an electrode is. Explain Electrode In Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Explain Electrode In Chemistry If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. That’s why scientists refer to metals as conductors: A semiconductor, an electrolyte, a vacuum or. electrochemical cells allow measurement and control of a redox reaction. electrochemistry is the study of chemical processes that. Explain Electrode In Chemistry.
From www.youtube.com
IonSelective Electrode, Principle, Advantages, Disadvantages Explain Electrode In Chemistry an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A semiconductor, an electrolyte, a vacuum or. — metals offer a great path. But to move electricity through non. electrochemistry is the study of chemical processes that cause electrons to move. electrodes are vital components of electrochemical cells.. Explain Electrode In Chemistry.
From www.alamy.com
Electrolysis hires stock photography and images Alamy Explain Electrode In Chemistry This movement of electrons is called electricity, which. electrochemistry is the study of chemical processes that cause electrons to move. electrochemical cells allow measurement and control of a redox reaction. That’s why scientists refer to metals as conductors: — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit.. Explain Electrode In Chemistry.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Explain Electrode In Chemistry electrochemical cells allow measurement and control of a redox reaction. electrochemistry is the study of chemical processes that cause electrons to move. But to move electricity through non. — metals offer a great path. A semiconductor, an electrolyte, a vacuum or. an electrode is an electrical conductor used to make contact with a nonmetallic part of. Explain Electrode In Chemistry.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Explain Electrode In Chemistry electrochemical cells allow measurement and control of a redox reaction. This movement of electrons is called electricity, which. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. But to move electricity through non. electrodes are vital components of electrochemical cells. If we place a variable resistance in. Explain Electrode In Chemistry.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Explain Electrode In Chemistry electrochemical cells allow measurement and control of a redox reaction. The reaction can be started and stopped by connecting or disconnecting the two electrodes. But to move electricity through non. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. That’s why scientists refer to metals as conductors: electrochemistry. Explain Electrode In Chemistry.
From thechemistrynotes.com
Electrode Potential Types, Importance, and Applications Explain Electrode In Chemistry — metals offer a great path. The reaction can be started and stopped by connecting or disconnecting the two electrodes. electrochemistry is the study of chemical processes that cause electrons to move. That’s why scientists refer to metals as conductors: A semiconductor, an electrolyte, a vacuum or. — an electrode can be defined as the point where. Explain Electrode In Chemistry.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Explain Electrode In Chemistry electrodes are vital components of electrochemical cells. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. electrochemistry is the study of chemical processes. Explain Electrode In Chemistry.
From www.shutterstock.com
926 Chemistry Electrodes Images, Stock Photos, 3D objects, & Vectors Explain Electrode In Chemistry But to move electricity through non. electrochemistry is the study of chemical processes that cause electrons to move. electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. — metals offer a. Explain Electrode In Chemistry.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Explain Electrode In Chemistry an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A semiconductor, an electrolyte, a vacuum or. The reaction can be started and stopped by connecting or disconnecting the two electrodes. That’s why scientists refer to metals as conductors: If we place a variable resistance in the circuit, we can even. Explain Electrode In Chemistry.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry Explain Electrode In Chemistry If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. But to move electricity through non. This movement of electrons is called electricity, which. electrochemical cells allow measurement and control of a redox reaction. an electrode is an electrical conductor used to make. Explain Electrode In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Explain Electrode In Chemistry A semiconductor, an electrolyte, a vacuum or. But to move electricity through non. electrochemical cells allow measurement and control of a redox reaction. electrodes are vital components of electrochemical cells. That’s why scientists refer to metals as conductors: If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction. Explain Electrode In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Explain Electrode In Chemistry That’s why scientists refer to metals as conductors: electrochemistry is the study of chemical processes that cause electrons to move. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A semiconductor, an electrolyte, a vacuum or. — an electrode can be defined as the point where current either. Explain Electrode In Chemistry.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Explain Electrode In Chemistry — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. But to move electricity through non. electrochemistry is the study of chemical processes that cause electrons to move. electrochemical cells allow measurement and control of a redox reaction. electrodes are vital components of electrochemical cells. an. Explain Electrode In Chemistry.
From www.toppr.com
Describe the construction and working of calomel electrode. Explain Electrode In Chemistry But to move electricity through non. — metals offer a great path. A semiconductor, an electrolyte, a vacuum or. That’s why scientists refer to metals as conductors: electrochemistry is the study of chemical processes that cause electrons to move. The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable. Explain Electrode In Chemistry.
From 2012books.lardbucket.org
Electrochemistry Explain Electrode In Chemistry If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. The reaction can be started and stopped by connecting or disconnecting the two electrodes. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. This. Explain Electrode In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Explain Electrode In Chemistry — metals offer a great path. This movement of electrons is called electricity, which. But to move electricity through non. electrochemistry is the study of chemical processes that cause electrons to move. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. That’s why scientists refer to metals as. Explain Electrode In Chemistry.
From chem.libretexts.org
20.2 Standard Electrode Potentials Chemistry LibreTexts Explain Electrode In Chemistry electrodes are vital components of electrochemical cells. That’s why scientists refer to metals as conductors: A semiconductor, an electrolyte, a vacuum or. This movement of electrons is called electricity, which. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. — metals offer a great path. The reaction. Explain Electrode In Chemistry.
From www.meritnation.com
Which Diagram should be drawn in Boards for a Standard Calomel Explain Electrode In Chemistry The reaction can be started and stopped by connecting or disconnecting the two electrodes. This movement of electrons is called electricity, which. electrochemistry is the study of chemical processes that cause electrons to move. But to move electricity through non. — metals offer a great path. — an electrode can be defined as the point where current. Explain Electrode In Chemistry.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Explain Electrode In Chemistry A semiconductor, an electrolyte, a vacuum or. — an electrode can be defined as the point where current either enters or leaves the electrolyte or circuit. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. electrodes are vital components of electrochemical cells. If we place a variable resistance. Explain Electrode In Chemistry.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Explain Electrode In Chemistry A semiconductor, an electrolyte, a vacuum or. — metals offer a great path. That’s why scientists refer to metals as conductors: This movement of electrons is called electricity, which. electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance in the circuit, we can even control the rate of the net cell. Explain Electrode In Chemistry.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Explain Electrode In Chemistry electrochemical cells allow measurement and control of a redox reaction. electrochemistry is the study of chemical processes that cause electrons to move. That’s why scientists refer to metals as conductors: This movement of electrons is called electricity, which. an electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. A. Explain Electrode In Chemistry.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Explain Electrode In Chemistry The reaction can be started and stopped by connecting or disconnecting the two electrodes. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. electrodes are vital components of electrochemical cells. electrochemistry is the study of chemical processes that cause electrons to move.. Explain Electrode In Chemistry.