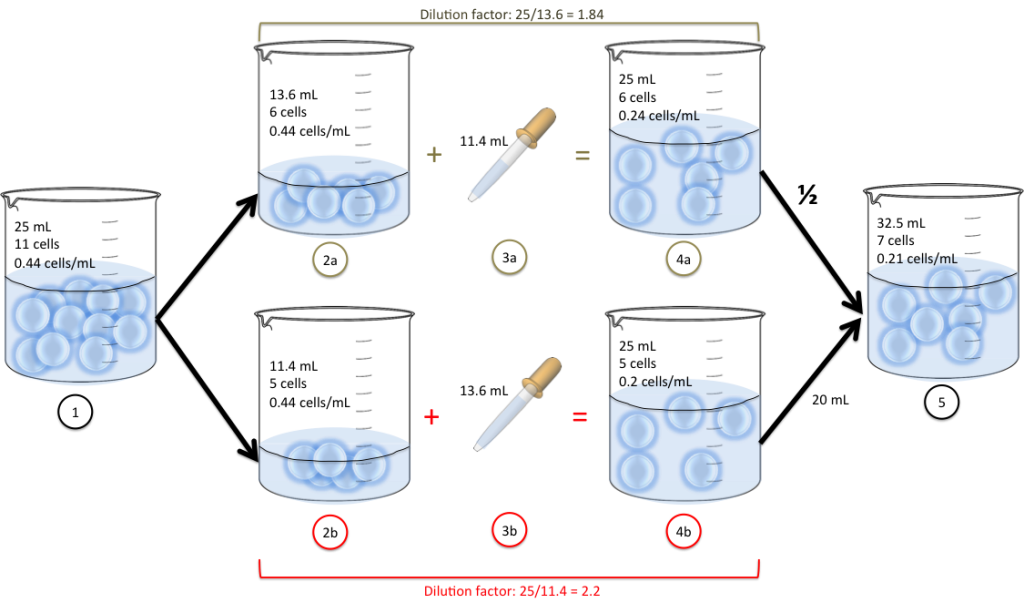
Dilution Factor Calculation Examples . How to calculate dilution factor. You can always check your results with our dilution factor calculator or just use it in the first place. Find any two of the following three. Here are a couple of examples: Below mentioned are the steps to calculate the dilution factor by hand: State whether the concentration of a solution is directly or indirectly proportional to its volume. Can you provide some examples of dilution factor calculation? Mixing 0.1 ml of a. Dilution factor calculations tutorial with worked examples for chemistry students The dilution factor is the ratio of the final volume to the initial volume. It encapsulates the essence of how much the original solution is diluted to. The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? It works either to find the dilution factor or the volume required to achieve. V f = aliquot volume.
from www.hemocytometer.org
What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? State whether the concentration of a solution is directly or indirectly proportional to its volume. How to calculate dilution factor. Below mentioned are the steps to calculate the dilution factor by hand: The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. V f = aliquot volume. Find any two of the following three. Here are a couple of examples: The dilution factor is the ratio of the final volume to the initial volume. It encapsulates the essence of how much the original solution is diluted to.
Using the dilution factor to calculate dilutions • Hemocytometer
Dilution Factor Calculation Examples Find any two of the following three. The dilution factor is the ratio of the final volume to the initial volume. Find any two of the following three. It encapsulates the essence of how much the original solution is diluted to. How to calculate dilution factor. V f = aliquot volume. Here are a couple of examples: It works either to find the dilution factor or the volume required to achieve. The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. Can you provide some examples of dilution factor calculation? State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first place. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Below mentioned are the steps to calculate the dilution factor by hand: Dilution factor calculations tutorial with worked examples for chemistry students Mixing 0.1 ml of a.
From www.scribd.com
To Calculate A Dilution Factor EXAMPLE What Is The Dilution Factor Dilution Factor Calculation Examples Dilution factor calculations tutorial with worked examples for chemistry students The dilution factor is the ratio of the final volume to the initial volume. Find any two of the following three. How to calculate dilution factor. It works either to find the dilution factor or the volume required to achieve. Can you provide some examples of dilution factor calculation? State. Dilution Factor Calculation Examples.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Calculation Examples Dilution factor calculations tutorial with worked examples for chemistry students Mixing 0.1 ml of a. V f = aliquot volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Can you provide some examples. Dilution Factor Calculation Examples.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Factor Calculation Examples How to calculate dilution factor. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Here are a couple of examples: It encapsulates the essence of how much the original solution is diluted to. The dilution factor (df) can be used alone or as the denominator of the fraction, for. Dilution Factor Calculation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Calculation Examples What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Below mentioned are the steps to calculate the dilution factor by hand: Here are a couple of examples: The dilution factor is the ratio of the final volume to the initial volume. Can you provide some examples of dilution factor. Dilution Factor Calculation Examples.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilution Factor Calculation Examples Can you provide some examples of dilution factor calculation? How to calculate dilution factor. The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. The dilution factor is the ratio of the final volume to the initial volume. Here are a couple of. Dilution Factor Calculation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Calculation Examples Dilution factor calculations tutorial with worked examples for chemistry students Below mentioned are the steps to calculate the dilution factor by hand: Mixing 0.1 ml of a. How to calculate dilution factor. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the dilution factor if you add a 0.1 ml aliquot of. Dilution Factor Calculation Examples.
From www.scribd.com
DilutionFactor Dilution Factor Calculation Examples Dilution factor calculations tutorial with worked examples for chemistry students Mixing 0.1 ml of a. Here are a couple of examples: Can you provide some examples of dilution factor calculation? It encapsulates the essence of how much the original solution is diluted to. You can always check your results with our dilution factor calculator or just use it in the. Dilution Factor Calculation Examples.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Calculation Examples Mixing 0.1 ml of a. Below mentioned are the steps to calculate the dilution factor by hand: Dilution factor calculations tutorial with worked examples for chemistry students What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Can you provide some examples of dilution factor calculation? It works either to. Dilution Factor Calculation Examples.
From www.youtube.com
Calculating Dilution Factor YouTube Dilution Factor Calculation Examples You can always check your results with our dilution factor calculator or just use it in the first place. The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. The dilution factor is the ratio of the final volume to the initial volume.. Dilution Factor Calculation Examples.
From www.slideshare.net
Molarity and dilution Dilution Factor Calculation Examples Dilution factor calculations tutorial with worked examples for chemistry students Here are a couple of examples: Mixing 0.1 ml of a. Can you provide some examples of dilution factor calculation? How to calculate dilution factor. It works either to find the dilution factor or the volume required to achieve. The dilution factor (df) can be used alone or as the. Dilution Factor Calculation Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Factor Calculation Examples What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. It works either to find the dilution factor or the volume required to. Dilution Factor Calculation Examples.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Dilution Factor Calculation Examples Here are a couple of examples: It works either to find the dilution factor or the volume required to achieve. Below mentioned are the steps to calculate the dilution factor by hand: It encapsulates the essence of how much the original solution is diluted to. Find any two of the following three. State whether the concentration of a solution is. Dilution Factor Calculation Examples.
From cfmuvi.jimdo.com
Simple Serial Dilution Calculation cfmuvi Dilution Factor Calculation Examples The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. Dilution factor calculations tutorial with worked examples for chemistry students V f = aliquot volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. How to calculate. Dilution Factor Calculation Examples.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Factor Calculation Examples The dilution factor is the ratio of the final volume to the initial volume. V f = aliquot volume. State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first place. Find any two of the following three.. Dilution Factor Calculation Examples.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Factor Calculation Examples Can you provide some examples of dilution factor calculation? Dilution factor calculations tutorial with worked examples for chemistry students Here are a couple of examples: State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent?. Dilution Factor Calculation Examples.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Factor Calculation Examples The dilution factor is the ratio of the final volume to the initial volume. It works either to find the dilution factor or the volume required to achieve. Mixing 0.1 ml of a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Below mentioned are the steps to calculate the dilution factor by hand:. Dilution Factor Calculation Examples.
From www.researchgate.net
The sample, dilution factor, and BOD values and the calculated degrees Dilution Factor Calculation Examples State whether the concentration of a solution is directly or indirectly proportional to its volume. It works either to find the dilution factor or the volume required to achieve. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Below mentioned are the steps to calculate the dilution factor by. Dilution Factor Calculation Examples.
From www.studocu.com
Calculating Dilution Factors Worksheet(1) Tagged How to Perform Dilution Factor Calculation Examples The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. Find any two of the following three. It encapsulates the essence of how much the original solution is diluted to. Below mentioned are the steps to calculate the dilution factor by hand: Dilution. Dilution Factor Calculation Examples.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Calculation Examples Below mentioned are the steps to calculate the dilution factor by hand: Dilution factor calculations tutorial with worked examples for chemistry students The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. Here are a couple of examples: State whether the concentration of. Dilution Factor Calculation Examples.
From lasopamail784.weebly.com
Calculate Dilution Factor lasopamail Dilution Factor Calculation Examples Here are a couple of examples: State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first place. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent?. Dilution Factor Calculation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Calculation Examples It encapsulates the essence of how much the original solution is diluted to. It works either to find the dilution factor or the volume required to achieve. You can always check your results with our dilution factor calculator or just use it in the first place. Dilution factor calculations tutorial with worked examples for chemistry students Mixing 0.1 ml of. Dilution Factor Calculation Examples.
From 134.209.152.22
Online Dilution Factor Calculator How to Calculate Dilution Factor Dilution Factor Calculation Examples Can you provide some examples of dilution factor calculation? It encapsulates the essence of how much the original solution is diluted to. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? It works either. Dilution Factor Calculation Examples.
From hresaje.weebly.com
Calculate Dilution Factor hresaje Dilution Factor Calculation Examples Here are a couple of examples: Can you provide some examples of dilution factor calculation? Find any two of the following three. You can always check your results with our dilution factor calculator or just use it in the first place. It works either to find the dilution factor or the volume required to achieve. What is the dilution factor. Dilution Factor Calculation Examples.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Factor Calculation Examples How to calculate dilution factor. The dilution factor is the ratio of the final volume to the initial volume. The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. V f = aliquot volume. It encapsulates the essence of how much the original. Dilution Factor Calculation Examples.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Factor Calculation Examples Mixing 0.1 ml of a. Below mentioned are the steps to calculate the dilution factor by hand: V f = aliquot volume. Can you provide some examples of dilution factor calculation? It encapsulates the essence of how much the original solution is diluted to. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to. Dilution Factor Calculation Examples.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Factor Calculation Examples You can always check your results with our dilution factor calculator or just use it in the first place. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? Find any two of the following three. State whether the concentration of a solution is directly or indirectly proportional to its. Dilution Factor Calculation Examples.
From www.youtube.com
Calculate dilution factor and concentration when diluting liquid and Dilution Factor Calculation Examples State whether the concentration of a solution is directly or indirectly proportional to its volume. Mixing 0.1 ml of a. V f = aliquot volume. Here are a couple of examples: It encapsulates the essence of how much the original solution is diluted to. How to calculate dilution factor. The dilution factor (df) can be used alone or as the. Dilution Factor Calculation Examples.
From factorcalculators.com
What is Dilution Factor? It's Examples, Definition and How do you Dilution Factor Calculation Examples Find any two of the following three. What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? V f = aliquot volume. Can you provide some examples of dilution factor calculation? Dilution factor calculations tutorial with worked examples for chemistry students You can always check your results with our dilution. Dilution Factor Calculation Examples.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Factor Calculation Examples What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? V f = aliquot volume. Below mentioned are the steps to calculate the dilution factor by hand: It works either to find the dilution factor or the volume required to achieve. Find any two of the following three. Dilution factor. Dilution Factor Calculation Examples.
From www.youtube.com
Dilutions using Dilution Factor Bio25 YouTube Dilution Factor Calculation Examples The dilution factor is the ratio of the final volume to the initial volume. Here are a couple of examples: Can you provide some examples of dilution factor calculation? How to calculate dilution factor. It works either to find the dilution factor or the volume required to achieve. Find any two of the following three. Below mentioned are the steps. Dilution Factor Calculation Examples.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Calculation Examples Find any two of the following three. Below mentioned are the steps to calculate the dilution factor by hand: It encapsulates the essence of how much the original solution is diluted to. The dilution factor is the ratio of the final volume to the initial volume. Mixing 0.1 ml of a. Can you provide some examples of dilution factor calculation?. Dilution Factor Calculation Examples.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Factor Calculation Examples Can you provide some examples of dilution factor calculation? The dilution factor (df) can be used alone or as the denominator of the fraction, for example, a df of 10 means a 1:10 dilution, or 1. How to calculate dilution factor. It works either to find the dilution factor or the volume required to achieve. The dilution factor is the. Dilution Factor Calculation Examples.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Calculation Examples Find any two of the following three. How to calculate dilution factor. You can always check your results with our dilution factor calculator or just use it in the first place. It works either to find the dilution factor or the volume required to achieve. Here are a couple of examples: It encapsulates the essence of how much the original. Dilution Factor Calculation Examples.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Factor Calculation Examples V f = aliquot volume. How to calculate dilution factor. Find any two of the following three. Dilution factor calculations tutorial with worked examples for chemistry students State whether the concentration of a solution is directly or indirectly proportional to its volume. You can always check your results with our dilution factor calculator or just use it in the first. Dilution Factor Calculation Examples.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Factor Calculation Examples Can you provide some examples of dilution factor calculation? The dilution factor is the ratio of the final volume to the initial volume. Here are a couple of examples: What is the dilution factor if you add a 0.1 ml aliquot of a specimen to 9.9 ml of diluent? It works either to find the dilution factor or the volume. Dilution Factor Calculation Examples.