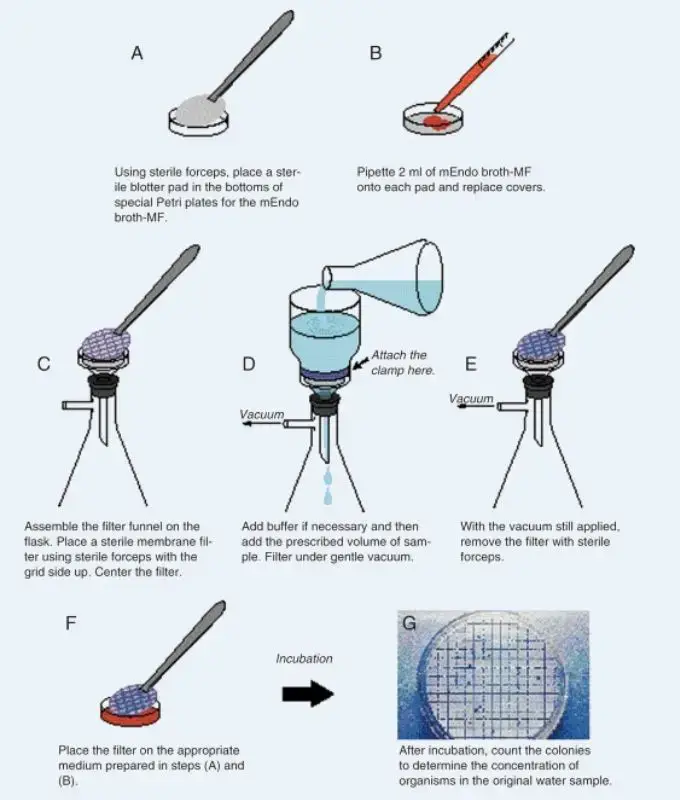
What Is Membrane Filtration Sterility Testing . Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Sterility testing is one of the most crucial steps in pharmaceutical product release. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The membrane filtration sterility test is the regulatory method.
from biologynotesonline.com
The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Sterility testing is one of the most crucial steps in pharmaceutical product release. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp.
Membrane Filtration Method Types, Advantages, Disadvantages
What Is Membrane Filtration Sterility Testing Sterility testing is one of the most crucial steps in pharmaceutical product release. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Sterility testing is one of the most crucial steps in pharmaceutical product release. The membrane filtration sterility test is the regulatory method. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where.
From solutionpharmacy.in
Sterility Test in Microbiology Solution Parmacy What Is Membrane Filtration Sterility Testing Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where.. What Is Membrane Filtration Sterility Testing.
From informacionpublica.svet.gob.gt
Filtration Sterilization Types, Mechanism, Uses What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The membrane filtration method is recommended for accommodating large volumes of test material or. What Is Membrane Filtration Sterility Testing.
From www.slideserve.com
PPT PHT 434 PowerPoint Presentation, free download ID3329067 What Is Membrane Filtration Sterility Testing Sterility testing is one of the most crucial steps in pharmaceutical product release. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The objective. What Is Membrane Filtration Sterility Testing.
From microbe-investigations.com
USP 71 Sterility Testing Services Microbe Investigations What Is Membrane Filtration Sterility Testing The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The membrane. What Is Membrane Filtration Sterility Testing.
From www.cobetterfiltration.com
Mixed Cellulose Esters (MCE) Membrane Filter OEM Membranes and What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Pharmacopeial. What Is Membrane Filtration Sterility Testing.
From www.pharmasopworld.com
Sterility Test validation What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Sterility testing is one of the most crucial steps in pharmaceutical product release. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration method. What Is Membrane Filtration Sterility Testing.
From worldcomplianceseminars.com
Microbiological Rapid Method Sterility Testing (RMT) of Short Dated What Is Membrane Filtration Sterility Testing The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Sterility testing. What Is Membrane Filtration Sterility Testing.
From www.emdmillipore.com
Membrane Filtration Sterility Test MilliporeSigma What Is Membrane Filtration Sterility Testing The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Sterility testing is one of the most crucial steps in pharmaceutical product release. Membrane filtration sterility. What Is Membrane Filtration Sterility Testing.
From www.researchgate.net
Membrane filtration process Download Scientific Diagram What Is Membrane Filtration Sterility Testing Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Pharmacopeial articles are. What Is Membrane Filtration Sterility Testing.
From www.emdmillipore.com
Membrane Filtration Sterility Test MilliporeSigma What Is Membrane Filtration Sterility Testing In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit.. What Is Membrane Filtration Sterility Testing.
From www.sartorius.hr
Sterility testing in accordance with the International Pharmacopoeia What Is Membrane Filtration Sterility Testing In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms.. What Is Membrane Filtration Sterility Testing.
From www.youtube.com
Sterility Testing Membrane Filtration YouTube What Is Membrane Filtration Sterility Testing Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration sterility. What Is Membrane Filtration Sterility Testing.
From www.youtube.com
Sterility testing closed membrane filtration method.....himedia YouTube What Is Membrane Filtration Sterility Testing Sterility testing is one of the most crucial steps in pharmaceutical product release. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. In membrane filtration, a certain quantity. What Is Membrane Filtration Sterility Testing.
From www.merckmillipore.com
Membrane Filtration Sterility Test Merck What Is Membrane Filtration Sterility Testing Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The objective of this validation is to establish documented evidence that the test for sterility. What Is Membrane Filtration Sterility Testing.
From mavink.com
Membrane Filtration Test What Is Membrane Filtration Sterility Testing Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The membrane filtration sterility. What Is Membrane Filtration Sterility Testing.
From www.scientistlive.com
Bringing the sterility test into the 21st Century Scientist Live What Is Membrane Filtration Sterility Testing The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The objective. What Is Membrane Filtration Sterility Testing.
From www.slideserve.com
PPT STERILITY TEST PowerPoint Presentation, free download ID1994580 What Is Membrane Filtration Sterility Testing In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Usp 71 sterility. What Is Membrane Filtration Sterility Testing.
From www.youtube.com
Microbiology 97 = Sterility Testing of Product Membrane Filtration What Is Membrane Filtration Sterility Testing The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. In membrane filtration, a. What Is Membrane Filtration Sterility Testing.
From www.labchromatography.com
what is membrane filtration sterility testingLab Chromatography Supplier What Is Membrane Filtration Sterility Testing Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The membrane filtration sterility test is the regulatory method. The objective of this validation is to establish documented evidence that the test for. What Is Membrane Filtration Sterility Testing.
From microbiologyclass.net
MEMBRANE FILTRATION TECHNIQUE 1 Microbiology Resource Hub What Is Membrane Filtration Sterility Testing Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Sterility testing is one of the most crucial steps in pharmaceutical product release. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The objective of this validation is to establish documented. What Is Membrane Filtration Sterility Testing.
From mavink.com
Membrane Filtration Test What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Sterility testing is one of the most crucial steps in pharmaceutical product release. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The. What Is Membrane Filtration Sterility Testing.
From www.emdmillipore.com
Membrane Filtration Sterility Test MilliporeSigma What Is Membrane Filtration Sterility Testing The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. In membrane filtration, a certain. What Is Membrane Filtration Sterility Testing.
From biologynotesonline.com
Membrane Filtration Method Types, Advantages, Disadvantages What Is Membrane Filtration Sterility Testing In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Sterility testing is one of the most crucial steps in pharmaceutical product release. The membrane filtration sterility test is the regulatory method. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited. What Is Membrane Filtration Sterility Testing.
From www.youtube.com
Sterility Testing Membrane Filtration & Direct Inoculation Method / L What Is Membrane Filtration Sterility Testing The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The membrane filtration sterility test is the regulatory method. The objective of this validation is to establish documented evidence that the test for sterility by membrane filtration method will produce the. Pharmacopeial articles are to be tested. What Is Membrane Filtration Sterility Testing.
From www.slideshare.net
Sterility tests What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. In membrane filtration, a certain quantity of a sterile product is filtered through two. What Is Membrane Filtration Sterility Testing.
From www.slideshare.net
Sterility test What Is Membrane Filtration Sterility Testing Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration sterility test is the regulatory method. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Sterility testing is one of the most crucial steps in pharmaceutical product release. The membrane filtration method is recommended. What Is Membrane Filtration Sterility Testing.
From www.slideshare.net
Sterility test What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. Usp 71 sterility testing helps ensure products labeled as sterile. What Is Membrane Filtration Sterility Testing.
From www.labchromatography.com
what is membrane filtration sterility testingLab Chromatography Supplier What Is Membrane Filtration Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration method is recommended. What Is Membrane Filtration Sterility Testing.
From www.labchromatography.com
what is membrane filtration sterility testingLab Chromatography Supplier What Is Membrane Filtration Sterility Testing The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. The membrane filtration sterility test is the regulatory method. Sterility testing is one of the. What Is Membrane Filtration Sterility Testing.
From www.slideshare.net
Sterility tests What Is Membrane Filtration Sterility Testing Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to be examined where. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. The membrane. What Is Membrane Filtration Sterility Testing.
From exomzvkxi.blob.core.windows.net
Membrane Filtration Method Can Be Used For Sterility Testing Of at What Is Membrane Filtration Sterility Testing The membrane filtration sterility test is the regulatory method. The membrane filtration method is recommended for accommodating large volumes of test material or when the test material contains substances which may inhibit. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. Usp 71 sterility testing helps ensure products labeled as. What Is Membrane Filtration Sterility Testing.
From www.slideserve.com
PPT STERILITY TEST PowerPoint Presentation, free download ID1994580 What Is Membrane Filtration Sterility Testing Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. In membrane filtration, a certain quantity of a sterile product is filtered through two canisters, each housing a membrane filter to trap. The membrane filtration sterility test is the regulatory method. Pharmacopeial articles are to be tested by the membrane filtration. What Is Membrane Filtration Sterility Testing.
From www.pinterest.com
MCE Micro Disc Membrane Filter, Sterile Gridded Individual Pack in 2022 What Is Membrane Filtration Sterility Testing Sterility testing is one of the most crucial steps in pharmaceutical product release. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to. What Is Membrane Filtration Sterility Testing.
From microbeonline.com
Membrane Filter Technique for Bacteriological Examination of Water What Is Membrane Filtration Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration sterility test is the regulatory method. Pharmacopeial articles are to be tested by the membrane filtration method under test for sterility of the product to. What Is Membrane Filtration Sterility Testing.
From procure-net.com
HTYAPL02 Sterility Test Pump Leading Innovation In Sterile Drug What Is Membrane Filtration Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Usp 71 sterility testing helps ensure products labeled as sterile are free from harmful microorganisms. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp. The membrane filtration sterility test is the regulatory method. The membrane filtration method. What Is Membrane Filtration Sterility Testing.