Phosphate Buffer How To Make . Because phosphoric acid has multiple. How to make pbs buffer. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Phosphate buffer is highly water soluble and. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Add 11.555 g of sodium phosphate dibasic to the solution. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add distilled water until the volume is 1 l. Recipe can be automatically scaled by entering desired final volume.
from animalia-life.club
Recipe can be automatically scaled by entering desired final volume. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. How to make pbs buffer. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Phosphate buffer is highly water soluble and. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How do you prepare your phosphate buffer? Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate.
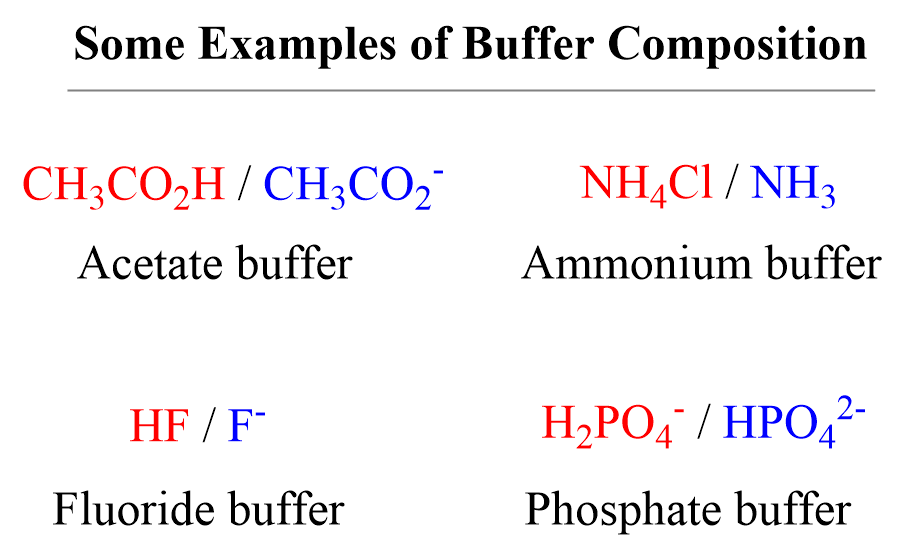
Phosphate Buffer System Equation
Phosphate Buffer How To Make How do you prepare your phosphate buffer? Phosphate buffer is highly water soluble and. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. How to make pbs buffer. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add distilled water until the volume is 1 l. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Because phosphoric acid has multiple. Add 11.555 g of sodium phosphate dibasic to the solution. How do you prepare your phosphate buffer? Recipe can be automatically scaled by entering desired final volume.
From 9to5science.com
[Solved] How can I prepare a PBS (Phosphate Buffer 9to5Science Phosphate Buffer How To Make Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. How do you prepare your phosphate buffer? Add distilled water until the volume is 1 l. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Add 11.555 g of sodium phosphate dibasic to the solution. Monosodium phosphate, monohydrate and its conjugate base disodium. Phosphate Buffer How To Make.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer How To Make Because phosphoric acid has multiple. How do you prepare your phosphate buffer? Recipe can be automatically scaled by entering desired final volume. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Weigh 10.9g. Phosphate Buffer How To Make.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer How To Make Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 11.555 g of sodium phosphate dibasic to the solution. How to make pbs buffer. Phosphate buffer is highly water soluble and. Add distilled water until the volume is 1 l. Because phosphoric acid has multiple. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about. Phosphate Buffer How To Make.
From www.scribd.com
How Do I Prepare a Phosphate Buffer Solution With a Specific PH Ph Phosphate Buffer How To Make A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Because phosphoric acid has multiple. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about. Phosphate Buffer How To Make.
From operaresidences.com.au
Describe the role of the phosphate buffer system? Opera Residences Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add distilled water until the volume is 1 l. Phosphate buffer is highly water soluble and. Because phosphoric acid has multiple. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. How to make pbs. Phosphate Buffer How To Make.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer How To Make Add 11.555 g of sodium phosphate dibasic to the solution. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Phosphate buffer is highly water soluble and. Recipe can be automatically scaled by entering desired final volume. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of. Phosphate Buffer How To Make.
From www.sliderbase.com
Acid and Base Balance and Imbalance Presentation Chemistry Phosphate Buffer How To Make Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. How do you prepare your phosphate buffer? Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Phosphate buffer (ph 5.8 to 7.4) preparation guide. Phosphate Buffer How To Make.
From www.youtube.com
phosphate buffer preparation protocol phosphate buffer as per usp Phosphate Buffer How To Make How to make pbs buffer. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How do you prepare your phosphate buffer? Add 11.555 g of sodium phosphate dibasic to the solution. Add distilled water until the volume is 1 l. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining. Phosphate Buffer How To Make.
From laboratorysales.com
PHOSPHATE BUFFER pH 7.2 1L Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. How to make pbs buffer. Because phosphoric acid has multiple. How do you prepare your phosphate buffer? Add distilled water until the volume is 1 l. Weigh 10.9g anhydrous sodium phosphate. Phosphate Buffer How To Make.
From studylib.net
Preparation Phosphate Buffer Saline (PBS) Phosphate Buffer How To Make Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Phosphate buffer is highly water soluble and. Add 11.555 g of sodium phosphate dibasic to the solution. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How to make pbs buffer. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Recipe can be. Phosphate Buffer How To Make.
From www.youtube.com
how to prepare a buffer with a particular pH YouTube Phosphate Buffer How To Make A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Recipe can be automatically scaled by entering desired final volume. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Add distilled water until the volume is 1 l.. Phosphate Buffer How To Make.
From www.weberscientific.com
Phosphate Buffer, pH 7.2 Preparation of dilution blanks. Phosphate Buffer How To Make Phosphate buffer is highly water soluble and. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Recipe can be automatically scaled by entering desired final volume. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized. Phosphate Buffer How To Make.
From bestonlinecollegesdegrees.com
Pbs Buffer Recipe Besto Blog Phosphate Buffer How To Make How to make pbs buffer. Recipe can be automatically scaled by entering desired final volume. Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Add distilled water until the volume is 1 l. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium.. Phosphate Buffer How To Make.
From www.wikihow.com
3 Ways to Make Phosphate Buffered Saline wikiHow Phosphate Buffer How To Make Because phosphoric acid has multiple. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How do you prepare your phosphate buffer? Phosphate buffer is highly water soluble and. Recipe can be automatically scaled by entering desired final volume. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml. Phosphate Buffer How To Make.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation ID2841454 Phosphate Buffer How To Make Recipe can be automatically scaled by entering desired final volume. Add distilled water until the volume is 1 l. Phosphate buffer is highly water soluble and. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. A phosphate buffer solution is. Phosphate Buffer How To Make.
From www.chegg.com
Solved Preparation of Phosphate Buffer Rxn H3PO4 H + H2PO4 Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add distilled water until the volume is 1 l. Because phosphoric acid has multiple. Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. A phosphate buffer solution is a handy. Phosphate Buffer How To Make.
From www.researchgate.net
Comparison of reactions in sodium phosphate buffer (pH 7.0) and Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add distilled water until the volume is 1 l. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Phosphate. Phosphate Buffer How To Make.
From www.thebalance.com
Guide to Making a Simple Phosphate Buffer Phosphate Buffer How To Make Recipe can be automatically scaled by entering desired final volume. Because phosphoric acid has multiple. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Add 11.555 g of sodium phosphate dibasic to the solution. How do you prepare. Phosphate Buffer How To Make.
From www.youtube.com
How to make Phosphate buffer 6.8 pH buffer 6.8 कैसे बनाये Phosphate Buffer How To Make Phosphate buffer is highly water soluble and. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. How do you prepare your phosphate buffer? Phosphate buffer (ph 5.8 to. Phosphate Buffer How To Make.
From www.slideserve.com
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11 Phosphate Buffer How To Make Add distilled water until the volume is 1 l. How to make pbs buffer. Phosphate buffer is highly water soluble and. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. How do you prepare your phosphate buffer? The phosphate buffer. Phosphate Buffer How To Make.
From www.researchgate.net
How can I prepare a potassium phosphate buffer 0.05M and pH 6 Phosphate Buffer How To Make Recipe can be automatically scaled by entering desired final volume. How to make pbs buffer. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add distilled water until the volume is 1 l. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4),. Phosphate Buffer How To Make.
From fdneurotech.com
0.1 M Phosphate Buffer, pH 7.4 FD Neuro Technologies, Inc. Phosphate Buffer How To Make A phosphate buffer solution is a handy buffer to have around, especially for biological applications. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Recipe can be automatically scaled by. Phosphate Buffer How To Make.
From www.researchgate.net
1b. Preparation of 0.1 M sodium phosphate 10× buffer at 25°C. a Phosphate Buffer How To Make Add distilled water until the volume is 1 l. Phosphate buffer is highly water soluble and. Recipe can be automatically scaled by entering desired final volume. How do you prepare your phosphate buffer? A phosphate buffer solution is a handy buffer to have around, especially for biological applications. How to make pbs buffer. Phosphate buffer (ph 5.8 to 7.4) preparation. Phosphate Buffer How To Make.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID3590784 Phosphate Buffer How To Make How to make pbs buffer. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Phosphate buffer is highly water soluble and. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Recipe can be automatically scaled by entering desired final volume. Phosphate buffer (ph. Phosphate Buffer How To Make.
From bestonlinecollegesdegrees.com
Pbs Buffer Recipe Besto Blog Phosphate Buffer How To Make Because phosphoric acid has multiple. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Add 11.555 g of sodium phosphate dibasic to the solution. Recipe can be automatically scaled by entering desired final volume. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Phosphate. Phosphate Buffer How To Make.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Phosphate buffer is highly water soluble and. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Add 11.555 g of sodium phosphate dibasic to the solution. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate,. Phosphate Buffer How To Make.
From www.bio-world.com
Sodium Phosphate Buffer 0.2M, pH 7.5 (7601549) bioWORLD Phosphate Buffer How To Make Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 11.555 g of sodium phosphate dibasic to the solution. How to make pbs buffer. Because phosphoric acid has multiple. Recipe can be automatically scaled by entering desired final volume. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Add distilled water until the volume is 1 l. Phosphate. Phosphate Buffer How To Make.
From www.chegg.com
Solved 19) Phosphate buffer solutions provide a basis of Phosphate Buffer How To Make Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Because phosphoric acid. Phosphate Buffer How To Make.
From www.slideserve.com
PPT Experiment No 3 PowerPoint Presentation, free download ID4828328 Phosphate Buffer How To Make Because phosphoric acid has multiple. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. How to make pbs buffer. Phosphate buffer is highly water soluble and. Recipe can be automatically scaled by entering desired final volume. Add 11.555 g of sodium phosphate dibasic. Phosphate Buffer How To Make.
From solvedlib.com
Phosphate buffer5 mL of 1M monobasic phosphate5 mL of… SolvedLib Phosphate Buffer How To Make Add distilled water until the volume is 1 l. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800. Phosphate Buffer How To Make.
From www.numerade.com
SOLVED A. Phosphate Buffer 1. Prepare 100 mL of a 0.01 M phosphate Phosphate Buffer How To Make How do you prepare your phosphate buffer? Because phosphoric acid has multiple. A phosphate buffer solution is a handy buffer to have around, especially for biological applications. Add 11.555 g of sodium phosphate dibasic to the solution. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology. Phosphate Buffer How To Make.
From studylib.net
phosphate buffer preparation of 0.1 M potassium phosphate buffer at Phosphate Buffer How To Make In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add distilled water until the volume is 1 l. How do you prepare your phosphate buffer? A phosphate buffer solution is a handy buffer to have around, especially for biological applications.. Phosphate Buffer How To Make.
From bio-solution.co.kr
[BS021] 0.5M Sodium Phosphate Buffer, pH x.x Biosolution Phosphate Buffer How To Make How do you prepare your phosphate buffer? Add distilled water until the volume is 1 l. The phosphate buffer solution is most commonly prepared at ph 7.2 for hematology staining as diluents for giemsa or leishman stain, using the monosodium and disodium. Phosphate buffer (ph 5.8 to 7.4) preparation guide and recipe. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g. Phosphate Buffer How To Make.
From dean-has-cooper.blogspot.com
How to Make Sodium Phosphate Buffer Ph 8 DeanhasCooper Phosphate Buffer How To Make How do you prepare your phosphate buffer? Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Add 11.555 g of sodium phosphate dibasic to the solution. In a clean beaker, dissolve 1.74 g of na2hpo4 (dibasic sodium phosphate) in about 800 ml of deionized water. The phosphate buffer solution is most commonly prepared. Phosphate Buffer How To Make.
From dean-has-cooper.blogspot.com
How to Make Sodium Phosphate Buffer Ph 8 DeanhasCooper Phosphate Buffer How To Make Phosphate buffer is highly water soluble and. Weigh 10.9g anhydrous sodium phosphate dibasic (na2hpo4), 3.2g anhydrous sodium phosphate monobasic (nah2po4), and 90g sodium. Add distilled water until the volume is 1 l. Monosodium phosphate, monohydrate and its conjugate base disodium phosphate, heptahydrate. Add 11.555 g of sodium phosphate dibasic to the solution. In a clean beaker, dissolve 1.74 g of. Phosphate Buffer How To Make.