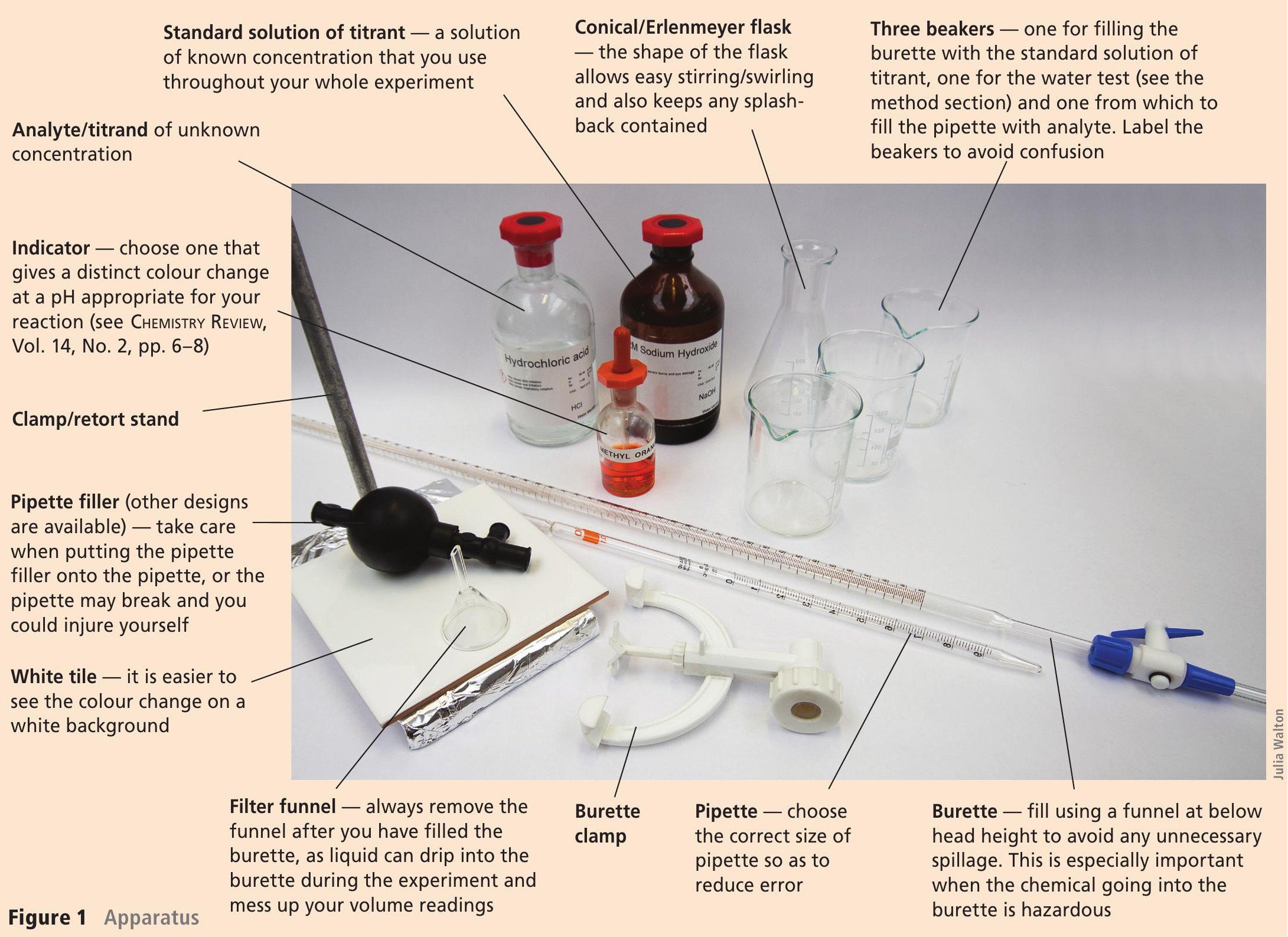
Titration Apparatus Definition . Measures the volume of an added solution accurately. Delivers an accurate volume of a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Generic apparatus for an acid base titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. The basic process involves adding a. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from www.hoddereducationmagazines.com
A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Generic apparatus for an acid base titration. The basic process involves adding a. Delivers an accurate volume of a solution. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Measures the volume of an added solution accurately.
Performing the perfect titration Hodder Education Magazines
Titration Apparatus Definition Delivers an accurate volume of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Generic apparatus for an acid base titration. The basic process involves adding a. Measures the volume of an added solution accurately. Delivers an accurate volume of a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Apparatus Definition The basic process involves adding a. Delivers an accurate volume of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration. Titration Apparatus Definition.
From www.alamy.com
Titration apparatus at chemical laboratory Stock Photo 29817654 Alamy Titration Apparatus Definition Measures the volume of an added solution accurately. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow. Titration Apparatus Definition.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Apparatus Definition Delivers an accurate volume of a solution. Measures the volume of an added solution accurately. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Generic apparatus for an acid base titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration Apparatus Definition.
From www.vrogue.co
Titration Illustration vrogue.co Titration Apparatus Definition Generic apparatus for an acid base titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a. Delivers an accurate volume of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is. Titration Apparatus Definition.
From www.savemyexams.com
Required Practical Strong Acid & Strong Alkali Titration AQA GCSE Chemistry Revision Notes 2018 Titration Apparatus Definition You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Generic apparatus for an acid base titration. Measures the volume of an added solution accurately. Delivers. Titration Apparatus Definition.
From acechemistry.co.uk
Preparing apparatus for a titration advanced Ace Chemistry Titration Apparatus Definition Measures the volume of an added solution accurately. The basic process involves adding a. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Delivers an accurate volume of a solution. You take a known volume of analyte (substance of unknown concentration) and. Titration Apparatus Definition.
From corinneafestuart.blogspot.com
Titration Apparatus Titration Apparatus Definition The basic process involves adding a. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Measures the volume of an added solution accurately. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding. Titration Apparatus Definition.
From techblog.ctgclean.com
Chemistry What is Titration? CTG Clean Titration Apparatus Definition Generic apparatus for an acid base titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Measures the volume of an added solution accurately. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Apparatus Definition.
From mavink.com
Titration Apparatus Diagram Titration Apparatus Definition You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Delivers an accurate volume of a solution. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration is. Titration Apparatus Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Apparatus Definition Generic apparatus for an acid base titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Measures. Titration Apparatus Definition.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Apparatus Definition Generic apparatus for an acid base titration. Delivers an accurate volume of a solution. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to. Titration Apparatus Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Apparatus Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow addition of. Titration Apparatus Definition.
From mavink.com
Titration Apparatus Diagram Titration Apparatus Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Generic apparatus for an acid base titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Measures the volume of an. Titration Apparatus Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Apparatus Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Measures the volume of an added solution accurately. The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured. Titration Apparatus Definition.
From mavink.com
Titration Apparatus Diagram Titration Apparatus Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Delivers an accurate volume of a solution. Measures the volume of an added solution. Titration Apparatus Definition.
From edu.rsc.org
Vintage titrations sulfur dioxide concentrations in wine Resource RSC Education Titration Apparatus Definition The basic process involves adding a. Delivers an accurate volume of a solution. Measures the volume of an added solution accurately. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. You take a known volume of analyte (substance of unknown concentration) and add a titrant. Titration Apparatus Definition.
From mavink.com
Titration Apparatus Diagram Titration Apparatus Definition The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Delivers an accurate volume of a solution. Generic apparatus for. Titration Apparatus Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Apparatus Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Generic apparatus for an acid base titration. Delivers an accurate volume of a solution. Measures the. Titration Apparatus Definition.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Apparatus Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Delivers an accurate volume of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Generic. Titration Apparatus Definition.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Apparatus Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Measures the volume of an added solution accurately. Generic apparatus for an acid base titration. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration. Titration Apparatus Definition.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali GCSE Chemistry Revision Titration Apparatus Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Generic apparatus for an acid base titration. The basic process involves adding a. Titration, process of chemical analysis. Titration Apparatus Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Apparatus Definition You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Generic apparatus for an acid base titration. The basic process involves adding a. Titration. Titration Apparatus Definition.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Apparatus Definition You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Measures the volume of an added solution accurately. The basic process involves adding a.. Titration Apparatus Definition.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Epharmacology HubPages Titration Apparatus Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Measures the volume of an added solution accurately. The basic process involves. Titration Apparatus Definition.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Apparatus Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Generic apparatus for an acid base titration. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. The basic process involves adding a. Measures. Titration Apparatus Definition.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Apparatus Definition Generic apparatus for an acid base titration. Measures the volume of an added solution accurately. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Apparatus Definition.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Point Titration Apparatus Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Measures the volume of an added solution accurately. Delivers an accurate volume of a solution. The basic process involves adding a. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Titration. Titration Apparatus Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Apparatus Definition The basic process involves adding a. Delivers an accurate volume of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Apparatus Definition.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Apparatus Definition Measures the volume of an added solution accurately. Delivers an accurate volume of a solution. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Apparatus Definition.
From www.frsscientific.com
Titration apparatus. STANDARD FRS Scientific Company Titration Apparatus Definition The basic process involves adding a. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of. Titration Apparatus Definition.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Exams Titration Apparatus Definition Delivers an accurate volume of a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Generic apparatus for an acid base titration. Measures the volume of an added solution accurately. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration. Titration Apparatus Definition.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Apparatus Definition Measures the volume of an added solution accurately. You take a known volume of analyte (substance of unknown concentration) and add a titrant (substance of know. Delivers an accurate volume of a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of. Titration Apparatus Definition.
From deven-jolpbloghancock.blogspot.com
Titration Apparatus Titration Apparatus Definition Measures the volume of an added solution accurately. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Generic apparatus for an acid base titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the. Titration Apparatus Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Apparatus Definition The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. A titration. Titration Apparatus Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Apparatus Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. The basic process involves adding a. Generic apparatus for an acid base titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration Apparatus Definition.