Terminal Define Chemistry . Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer molecule and a point at which electron. The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The terminal atoms simply provided the electrons needed by the central atom. It is used to show how the electrons are arranged around.
from www.masterorganicchemistry.com
Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. It is used to show how the electrons are arranged around. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer molecule and a point at which electron. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The terminal atoms simply provided the electrons needed by the central atom. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa).
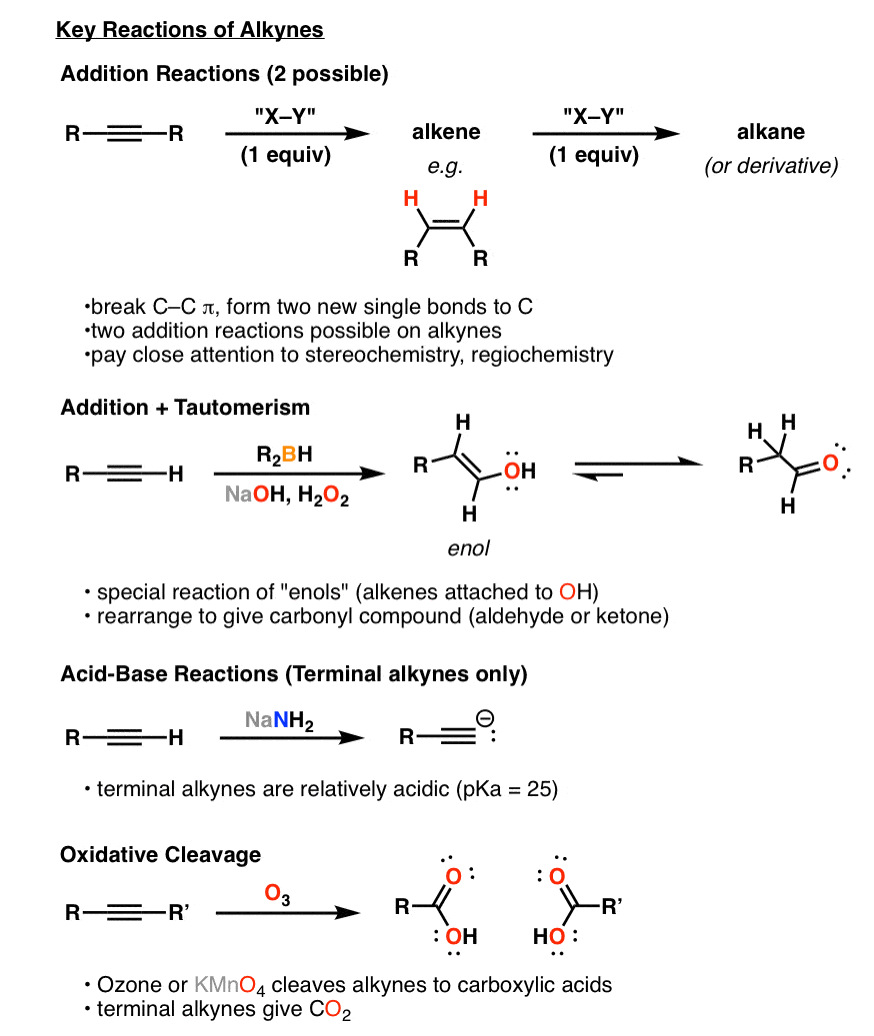
Synthesis (5) Reactions of Alkynes Master Organic Chemistry
Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. The terminal atoms simply provided the electrons needed by the central atom. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. It is used to show how the electrons are arranged around. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The end of a polymer molecule and a point at which electron.
From chem.libretexts.org
NTerminal Chemistry LibreTexts Terminal Define Chemistry The end of a polymer molecule and a point at which electron. The terminal atoms simply provided the electrons needed by the central atom. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer. Terminal Define Chemistry.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Terminal Define Chemistry The end of a polymer molecule and a point at which electron. The terminal atoms simply provided the electrons needed by the central atom. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). It is used to show how the electrons are arranged around. The end of a polymer molecule and a point at which. Terminal Define Chemistry.
From www.biologyonline.com
Axon terminal Definition and Examples Biology Online Dictionary Terminal Define Chemistry The end of a polymer molecule and a point at which electron. It is used to show how the electrons are arranged around. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The bottom line here is that by. Terminal Define Chemistry.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Terminal Define Chemistry It is used to show how the electrons are arranged around. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The end of a polymer molecule and a point at which electron connections can easily be made or broken.. Terminal Define Chemistry.
From dvn.com.vn
Meet the (Most Important) Functional Groups Chia Sẻ Kiến Thức Điện Terminal Define Chemistry Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. It is used to show how the electrons are arranged around. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron. The end of a polymer molecule. Terminal Define Chemistry.
From www.slideserve.com
PPT Output PowerPoint Presentation, free download ID537527 Terminal Define Chemistry The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The terminal atoms simply provided the electrons needed by the central atom. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). It is used to show. Terminal Define Chemistry.
From www.chegg.com
Chemistry Archive October 28, 2016 Terminal Define Chemistry A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The terminal atoms simply provided the electrons needed by the central atom. Learn about and revise electrolysis with. Terminal Define Chemistry.
From www.pinterest.co.uk
ALKYNES TERMINAL Organic chemistry, Teaching chemistry, Chemistry Terminal Define Chemistry The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer molecule and a point at which electron. The end of a polymer molecule and a point at which electron connections can easily be made or broken.. Terminal Define Chemistry.
From slideplayer.com
Chapter 8 The Nervous System ppt download Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. It is used to show how the electrons are arranged around. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the. Terminal Define Chemistry.
From www.mdpi.com
Molecules Free FullText Exploiting Protein NTerminus for Site Terminal Define Chemistry The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The terminal atoms simply provided the electrons needed by the central atom. The end of a polymer molecule and a point at which electron connections can easily be made or broken.. Terminal Define Chemistry.
From www.masterorganicchemistry.com
Synthesis (5) Reactions of Alkynes Master Organic Chemistry Terminal Define Chemistry The end of a polymer molecule and a point at which electron connections can easily be made or broken. It is used to show how the electrons are arranged around. The end of a polymer molecule and a point at which electron. The bottom line here is that by counting the number of radicals created or destroyed in each step,. Terminal Define Chemistry.
From chemistry-europe.onlinelibrary.wiley.com
Catalytic 1,3‐H Atom Shift of a Terminal Benzylic Alkyne by Iron and Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. It is used to show how the electrons are arranged around. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The bottom line here is that by counting the number of radicals created or destroyed in each step,. Terminal Define Chemistry.
From socratic.org
What is a voltaic cell? + Example Terminal Define Chemistry The end of a polymer molecule and a point at which electron. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer molecule and a point at which electron connections can easily be made or broken.. Terminal Define Chemistry.
From www.animalia-life.club
Neuron Labeled And Functions Terminal Define Chemistry A lewis structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around. The terminal atoms simply provided the electrons needed by the central atom. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The bottom. Terminal Define Chemistry.
From chemistry.stackexchange.com
organic chemistry why the terminal Br/Cl is an electrophile in AlBr5 Terminal Define Chemistry The end of a polymer molecule and a point at which electron. It is used to show how the electrons are arranged around. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The bottom line here is that by counting the number of radicals created or destroyed in each step,. Terminal Define Chemistry.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The bottom line here is that by counting the number of radicals created or destroyed in each. Terminal Define Chemistry.
From www.thoughtco.com
How to Define Anode and Cathode Terminal Define Chemistry The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The terminal atoms simply provided the electrons needed by the central atom. The bottom line here is that by counting the number of radicals created or. Terminal Define Chemistry.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Terminal Define Chemistry It is used to show how the electrons are arranged around. The terminal atoms simply provided the electrons needed by the central atom. The end of a polymer molecule and a point at which electron. The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about the production of voltage. Terminal Define Chemistry.
From www.pinterest.com
Electrolyte Easy Science Electrolytes, Easy science, Flashcards Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron. It is used to show how the electrons are arranged around. The end of a polymer molecule and a point. Terminal Define Chemistry.
From stansburycasted.blogspot.com
Chemistry 1 Formula Writting and Naming Continued Practice Extra Terminal Define Chemistry A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. Learn about the. Terminal Define Chemistry.
From www.cell.com
Protein NTerminal Acetylation Structural Basis, Mechanism Terminal Define Chemistry It is used to show how the electrons are arranged around. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The end of a polymer molecule and a point at which electron connections can easily be made or broken. The end of a polymer molecule and a point at which electron. The terminal atoms. Terminal Define Chemistry.
From www.researchgate.net
1. Chemical Cterminal sequence analysis using the alkylation Terminal Define Chemistry Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. It is used to show how the electrons are arranged around. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer. Terminal Define Chemistry.
From www.youtube.com
Structure of Atom Chemistry, Definition, Examples YouTube Terminal Define Chemistry A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. Learn about the production of voltage using chemical cells with. Terminal Define Chemistry.
From www.masterorganicchemistry.com
Synthesis of Peptides Master Organic Chemistry Terminal Define Chemistry The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The. Terminal Define Chemistry.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Terminal Define Chemistry The end of a polymer molecule and a point at which electron. The terminal atoms simply provided the electrons needed by the central atom. The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). It is used. Terminal Define Chemistry.
From animalia-life.club
Simple Synapse Diagram Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The end of a polymer molecule and a point at which electron connections can easily be made or broken. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa).. Terminal Define Chemistry.
From www.pinterest.com.mx
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Terminal Define Chemistry It is used to show how the electrons are arranged around. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. Learn about and revise. Terminal Define Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Nterminus Terminal Define Chemistry A lewis structure is a very simplified representation of the valence shell electrons in a molecule. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The end of a polymer molecule and a point at which electron connections can easily. Terminal Define Chemistry.
From www.gaylordchemical.com
DMSO / K2CO3 Terminal Alkyne Synthesis Gaylord Chemical Terminal Define Chemistry Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). It is used to show how the electrons are arranged around. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer molecule. Terminal Define Chemistry.
From www.mdpi.com
Molecules Free FullText Exploiting Protein NTerminus for Site Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). The bottom line here is that by counting the number of radicals created or destroyed in each step, you. Terminal Define Chemistry.
From helpfulprofessor.com
Terminal Values 10 Examples and Definition (2024) Terminal Define Chemistry Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. It is used to show how the electrons are arranged around. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. The end of a polymer. Terminal Define Chemistry.
From www.royalhaskoningdhv.com
Liquid bulk and chemical terminals Royal HaskoningDHV Terminal Define Chemistry It is used to show how the electrons are arranged around. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The end of a polymer molecule and a point at which electron. Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The bottom line here is that. Terminal Define Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Terminal Define Chemistry The end of a polymer molecule and a point at which electron connections can easily be made or broken. A lewis structure is a very simplified representation of the valence shell electrons in a molecule. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is. Terminal Define Chemistry.
From www.atlearner.com
What is an Electric Cell? Definition, Types of Cell Terminal Define Chemistry The terminal atoms simply provided the electrons needed by the central atom. Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The bottom line here is that by counting the number of radicals created or destroyed in each step, you. Terminal Define Chemistry.
From animalia-life.club
Simple Synapse Diagram Terminal Define Chemistry Learn about the production of voltage using chemical cells with gcse bitesize chemistry (aqa). Learn about and revise electrolysis with this bbc bitesize gcse chemistry (ocr 21c) study guide. The bottom line here is that by counting the number of radicals created or destroyed in each step, you can determine if the step is initiation, propagation, or termination. It is. Terminal Define Chemistry.