Vegetable Oil Formula Chemistry . Not only do they have about twice as many calories per gram as. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Find out how they differ in melting points, fatty acid content, and health effects. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects.
from www.alamy.com
This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Find out how they differ in melting points, fatty acid content, and health effects. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Not only do they have about twice as many calories per gram as. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils.
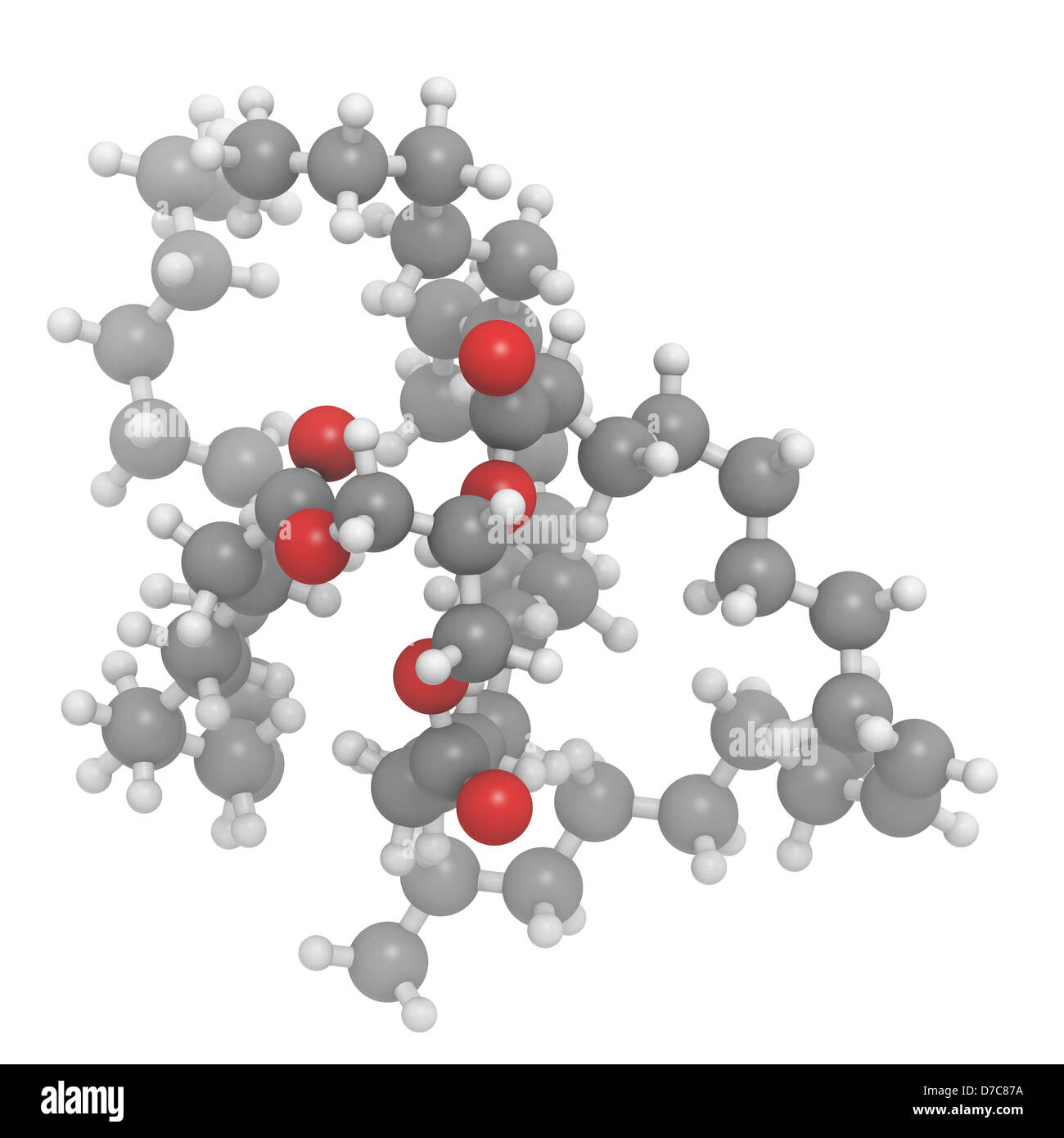
Vegetable oil unsaturated triglyceride molecule, chemical structure
Vegetable Oil Formula Chemistry Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Not only do they have about twice as many calories per gram as. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Find out how they differ in melting points, fatty acid content, and health effects. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils.
From andi-healthy.blogspot.com
Vegetable Oil Molecular Structure Andi Healthy Vegetable Oil Formula Chemistry Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their. Vegetable Oil Formula Chemistry.
From www.alamy.com
Vegetable oil unsaturated triglyceride molecule, chemical structure Vegetable Oil Formula Chemistry Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Not only do they have about twice. Vegetable Oil Formula Chemistry.
From www.alamy.com
Vegetable oil unsaturated triglyceride molecule, chemical structure Vegetable Oil Formula Chemistry In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about. Vegetable Oil Formula Chemistry.
From courses.lumenlearning.com
17.2 Fats and Oils The Basics of General, Organic, and Biological Vegetable Oil Formula Chemistry Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn how fats and. Vegetable Oil Formula Chemistry.
From courses.lumenlearning.com
17.2 Fats and Oils The Basics of General, Organic, and Biological Vegetable Oil Formula Chemistry Not only do they have about twice as many calories per gram as. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their. Vegetable Oil Formula Chemistry.
From cartoondealer.com
Elaidic Acid Molecule. The Main Trans Fat Found In Hydrogenated Vegetable Oil Formula Chemistry Find out how they differ in melting points, fatty acid content, and health effects. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Not only do they have about twice as many calories per gram as. Find out how hydrogenation, saturation, and unsaturation affect the melting. Vegetable Oil Formula Chemistry.
From animalia-life.club
Hydrogenated Oil Structure Vegetable Oil Formula Chemistry In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Dietary intake of vegetable. Vegetable Oil Formula Chemistry.
From pubs.rsc.org
State of the art of biodiesel production processes a review of the Vegetable Oil Formula Chemistry This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Learn about the structure, composition, and properties of fats and oils, the most. Vegetable Oil Formula Chemistry.
From www.dyseg.com
Hydrogenated Vegetable Oil Chemical Formula Best Vegetable In The World Vegetable Oil Formula Chemistry Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about twice as many calories per gram as. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical. Vegetable Oil Formula Chemistry.
From goshen.edu
The Chemistry of Biodiesel Biodiesel Project Goshen College Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. This web page is an. Vegetable Oil Formula Chemistry.
From courses.lumenlearning.com
Hydrogenation Introduction to Chemistry Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Find out how they differ in melting points, fatty acid. Vegetable Oil Formula Chemistry.
From ar.inspiredpencil.com
Vegetable Oil Structural Formula Vegetable Oil Formula Chemistry Find out how they differ in melting points, fatty acid content, and health effects. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Not only do they have about twice as many calories per gram as. In a reaction to form. Vegetable Oil Formula Chemistry.
From www.semanticscholar.org
Figure 8 from Canola Oil Physical and Chemical Properties by Vegetable Oil Formula Chemistry This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to. Vegetable Oil Formula Chemistry.
From www.researchgate.net
Composition of hydrogenated vegetable oils and vegetable oil Vegetable Oil Formula Chemistry Find out how they differ in melting points, fatty acid content, and health effects. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Dietary intake. Vegetable Oil Formula Chemistry.
From www.slideserve.com
PPT Introduction to Oil Chemistry and Transesterification PowerPoint Vegetable Oil Formula Chemistry Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about twice as many calories per gram as. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Find out how hydrogenation, saturation, and unsaturation affect the melting. Vegetable Oil Formula Chemistry.
From www.reddit.com
Found a pretty neat high quality image of saturated fatty acids r Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn about the structure, composition, and properties of fats and oils, the most. Vegetable Oil Formula Chemistry.
From courses.lumenlearning.com
17.2 Fats and Oils The Basics of General, Organic, and Biological Vegetable Oil Formula Chemistry Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of. Vegetable Oil Formula Chemistry.
From ar.inspiredpencil.com
Vegetable Oil Structure Vegetable Oil Formula Chemistry Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Find out how they differ in melting points, fatty acid content, and health effects. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Not only do they have about. Vegetable Oil Formula Chemistry.
From www.intechopen.com
Chemical Structure, Quality Indices and Bioactivity of Essential Oil Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. This web page is an extract from a book chapter. Vegetable Oil Formula Chemistry.
From animalia-life.club
Soybean Oil Structural Formula Vegetable Oil Formula Chemistry Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. This web page is an extract from a book chapter that provides data and analysis on the global trade and. Vegetable Oil Formula Chemistry.
From www.tessshebaylo.com
Chemical Equation For Making Biodiesel Tessshebaylo Vegetable Oil Formula Chemistry Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about twice as many calories per gram as. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. In a reaction to form biodiesel, one mole. Vegetable Oil Formula Chemistry.
From www.researchgate.net
Formulas and structures of the most common fatty acids in vegetable Vegetable Oil Formula Chemistry Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their. Vegetable Oil Formula Chemistry.
From animalia-life.club
Hydrogenated Oil Structure Vegetable Oil Formula Chemistry In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Not only do they have about twice as many calories per gram as. Dietary intake of vegetable oils. Vegetable Oil Formula Chemistry.
From www.dreamstime.com
Elaidic Acid Molecule. The Main Trans Fat Found In Hydrogenated Vegetable Oil Formula Chemistry In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Learn how fats and oils are triglycerides, esters of. Vegetable Oil Formula Chemistry.
From www.mdpi.com
Processes Free FullText NMR Determination of Free Fatty Acids in Vegetable Oil Formula Chemistry Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles. Vegetable Oil Formula Chemistry.
From www.dreamstime.com
Fatty Acid Composition of Olive Oil. Structural Chemical Formulas Stock Vegetable Oil Formula Chemistry In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Learn about the structure, composition, and. Vegetable Oil Formula Chemistry.
From www.shutterstock.com
Vegetable Oil Unsaturated Triglyceride Molecule Chemical Stock Vegetable Oil Formula Chemistry This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Not only do they have about twice as many calories per gram as. Find out how they differ in melting points, fatty acid content, and health effects. Learn about the structure, composition, and properties of fats. Vegetable Oil Formula Chemistry.
From www.slideserve.com
PPT VEGETABLE OILS A guide for GCSE students PowerPoint Presentation Vegetable Oil Formula Chemistry Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Not only do they have about twice as many calories per gram as. This web page is an extract from a book chapter that provides data and analysis on the global trade and use of. Vegetable Oil Formula Chemistry.
From www.alamy.com
Alphalinolenic acid, ALA molecule. Carboxylic, polyunsaturated omega3 Vegetable Oil Formula Chemistry This web page is an extract from a book chapter that provides data and analysis on the global trade and use of vegetable oils. Not only do they have about twice as many calories per gram as. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure.. Vegetable Oil Formula Chemistry.
From www.mdpi.com
IJMS Free FullText Fabrication of Silver Nanoparticles Dispersed Vegetable Oil Formula Chemistry Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their. Vegetable Oil Formula Chemistry.
From www.slideserve.com
PPT Chemistry 2100 PowerPoint Presentation, free download ID6574979 Vegetable Oil Formula Chemistry Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Find out how they differ in melting points,. Vegetable Oil Formula Chemistry.
From mybios.me
Chemical Position Of Vegetable Oil My Bios Vegetable Oil Formula Chemistry Dietary intake of vegetable oils and hydrogenated vegetable oils has significant health effects. Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about. Vegetable Oil Formula Chemistry.
From www.dreamstime.com
Oil composition stock vector. Illustration of ring, atoms 64171764 Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Learn about the structure, composition, and properties of fats and oils, the most abundant lipids in nature. Find out how they differ in melting points, fatty acid content, and health effects. Not only do they have about. Vegetable Oil Formula Chemistry.
From hxezumpbi.blob.core.windows.net
Vegetable Oil Molecular Formula at Doris Archibald blog Vegetable Oil Formula Chemistry Find out how hydrogenation, saturation, and unsaturation affect the melting point and. Learn about the chemical structure, properties, and types of fats and oils, including vegetable oils. In a reaction to form biodiesel, one mole of a vegetable oil reacts with an excess of methanol to form two moles of an ester with molecular. Dietary intake of vegetable oils and. Vegetable Oil Formula Chemistry.
From www.researchgate.net
Structure of hydrogenated castor oil produced from hydrogenation of Vegetable Oil Formula Chemistry Learn how fats and oils are triglycerides, esters of glycerol and fatty acids, and how their properties depend on their composition and structure. Not only do they have about twice as many calories per gram as. Find out how they differ in melting points, fatty acid content, and health effects. This web page is an extract from a book chapter. Vegetable Oil Formula Chemistry.