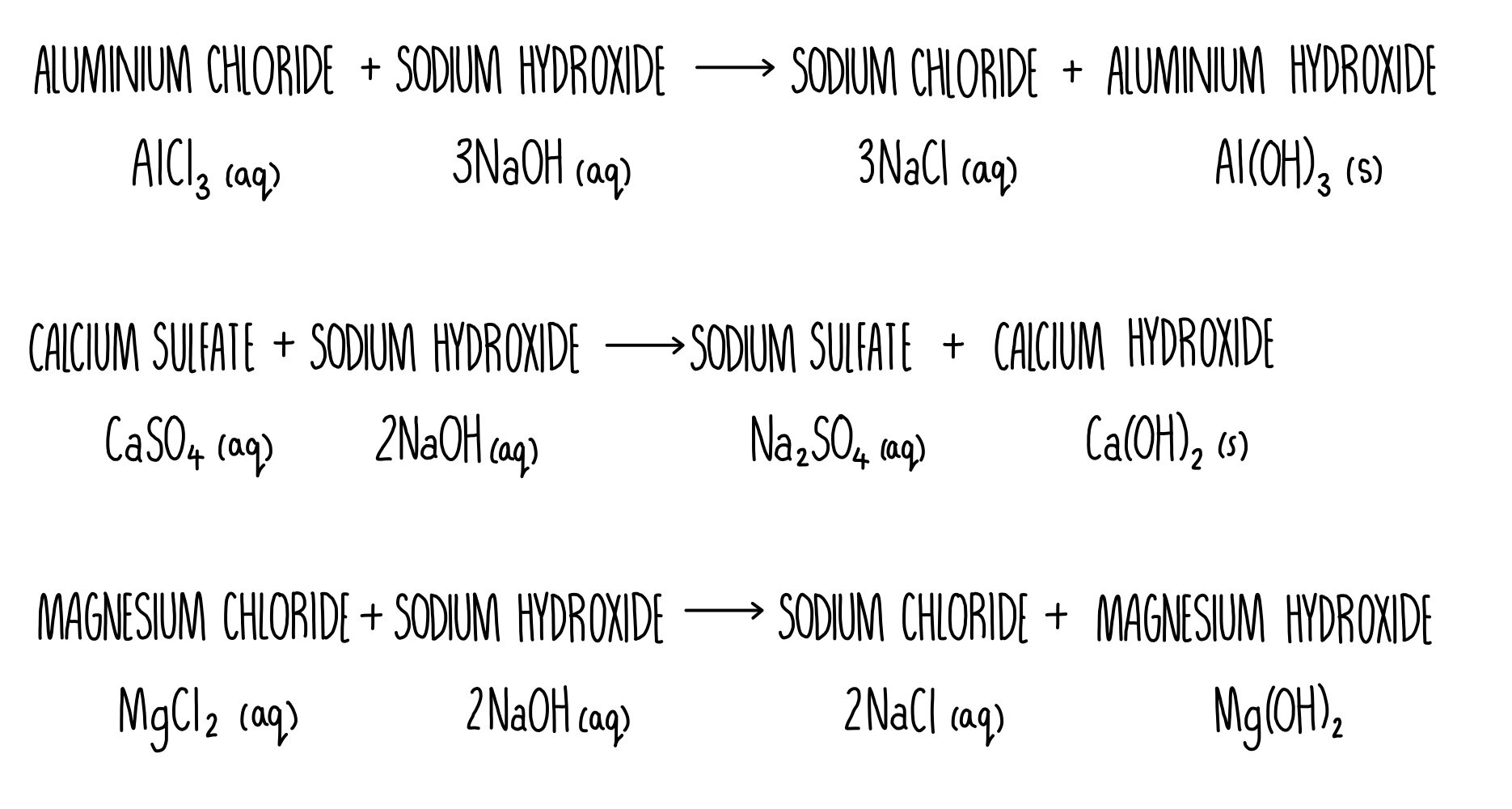
Aluminum Chloride And Sodium Hydroxide . aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. The aluminum hydroxide tends to cause.
from www.thesciencehive.co.uk
the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. The aluminum hydroxide tends to cause. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3.
Identifying Ions (AQA) — the science sauce
Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. The aluminum hydroxide tends to cause. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3.
From www.chegg.com
Solved Additional Problems 1. For each of the following, Aluminum Chloride And Sodium Hydroxide aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. in this video we'll balance the equation alcl3 + naoh = al. Aluminum Chloride And Sodium Hydroxide.
From www.slideserve.com
PPT Electrolysis An Introduction VCE Chemistry Unit 4 Chemistry at Aluminum Chloride And Sodium Hydroxide the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis). Aluminum Chloride And Sodium Hydroxide.
From mungfali.com
Structure Of Sodium Hydroxide Aluminum Chloride And Sodium Hydroxide in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. The aluminum hydroxide tends to cause. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. aluminium chloride, also known as. Aluminum Chloride And Sodium Hydroxide.
From insende.netlify.app
31++ Hydrochloric Acid And Sodium Hydroxide Formula Insende Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium.. Aluminum Chloride And Sodium Hydroxide.
From www.alamy.com
Sodium Hydroxide and Sodium Chloride Molecular Model of Atom. Vector Aluminum Chloride And Sodium Hydroxide aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. The aluminum hydroxide tends to cause. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. alcl3. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
Does Sodium hydroxide (NaOH) and Aluminium chloride (AlCl3) forms a Aluminum Chloride And Sodium Hydroxide aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. the balanced chemical equation for the reaction. Aluminum Chloride And Sodium Hydroxide.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide And Sodium Hydroxide Aluminum Chloride And Sodium Hydroxide in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. The aluminum hydroxide tends to cause. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. does sodium hydroxide (naoh) and. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
Write down the formulae of (i) sodium oxide , Aluminum chloride NCERT Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and.. Aluminum Chloride And Sodium Hydroxide.
From www.toppr.com
2. Write the balanced equation the following chemical reactions. (i Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. the balanced chemical equation for the reaction between aqueous solutions. Aluminum Chloride And Sodium Hydroxide.
From www.scribd.com
Aluminium Sodium Hydroxide Aluminium Oxide Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms.. Aluminum Chloride And Sodium Hydroxide.
From stock.adobe.com
Vetor de illustration of chemistry, Acids, salt and bases, Hydrogen Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. The aluminum hydroxide tends to cause. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. several antacids have aluminum hydroxide, al(oh). Aluminum Chloride And Sodium Hydroxide.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. . Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
Reaction of Aluminum with Water and Sodium Hydroxide YouTube Aluminum Chloride And Sodium Hydroxide in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. several antacids have aluminum hydroxide, al(oh) 3,. Aluminum Chloride And Sodium Hydroxide.
From fineartamerica.com
Aluminium Precipitate In Sodium Hydroxide Photograph by Martyn F Aluminum Chloride And Sodium Hydroxide The aluminum hydroxide tends to cause. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. alcl3 + naoh = alh3o3 + nacl. Aluminum Chloride And Sodium Hydroxide.
From www.numerade.com
SOLVED Write balanced equations for each of the following reactions Aluminum Chloride And Sodium Hydroxide The aluminum hydroxide tends to cause. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. in this video we'll balance the equation alcl3 + naoh = al (oh)3 +. Aluminum Chloride And Sodium Hydroxide.
From www.sciencephoto.com
Aluminium in excess sodium hydroxide Stock Image C019/9199 Aluminum Chloride And Sodium Hydroxide in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. several antacids have aluminum hydroxide, al(oh) 3, as an. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
How to Write the Net Ionic Equation for NH4Cl + NaOH = NaCl + H2O + NH3 Aluminum Chloride And Sodium Hydroxide does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. The aluminum hydroxide tends to cause. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis). Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide and Aluminum Foil YouTube Aluminum Chloride And Sodium Hydroxide the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. The aluminum hydroxide tends to cause. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. in. Aluminum Chloride And Sodium Hydroxide.
From www.numerade.com
SOLVED Ferric chloride is formed by the reaction of iron and chlorine Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. in this video we'll balance the equation alcl3 + naoh = al. Aluminum Chloride And Sodium Hydroxide.
From www.alamy.com
Sodium Hydroxide and Sodium Chloride Molecular Model of Atom. Vector Aluminum Chloride And Sodium Hydroxide aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. The. Aluminum Chloride And Sodium Hydroxide.
From www.thesciencehive.co.uk
Identifying Ions (AQA) — the science sauce Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. The aluminum hydroxide tends to cause. alcl3 + naoh = alh3o3 + nacl. Aluminum Chloride And Sodium Hydroxide.
From www.numerade.com
SOLVEDAqueous solutions of aluminum chloride and sodium hydroxide are Aluminum Chloride And Sodium Hydroxide does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. The aluminum hydroxide. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
How to Balance AlCl3 + NaOH = Al(OH)3 + NaCl Aluminum chloride Aluminum Chloride And Sodium Hydroxide The aluminum hydroxide tends to cause. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride.. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
TO STUDY THE REACTIONS OF SODIUM HYDROXIDE WITH ALUMINIUM METAL AND Aluminum Chloride And Sodium Hydroxide aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. The aluminum hydroxide tends to cause. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium chloride, also known as aluminium trichloride, is. Aluminum Chloride And Sodium Hydroxide.
From dxoalzrzs.blob.core.windows.net
Aluminum Chloride Vs Aluminium Chlorohydrate at Donald Miller blog Aluminum Chloride And Sodium Hydroxide in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. does sodium hydroxide (naoh) and aluminium chloride. Aluminum Chloride And Sodium Hydroxide.
From slideplayer.com
Types of Chemical Reactions ppt download Aluminum Chloride And Sodium Hydroxide The aluminum hydroxide tends to cause. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium chloride, also known as aluminium trichloride, is an inorganic. Aluminum Chloride And Sodium Hydroxide.
From rxmarine.com
Aluminium Hydroxide Chloride, solution Manufacturer, Supplier, Exporter Aluminum Chloride And Sodium Hydroxide aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. The aluminum hydroxide tends to cause. in this video we'll balance the equation alcl3 + naoh =. Aluminum Chloride And Sodium Hydroxide.
From www.toppr.com
Write formulae of the following compounds.Sodium phosphate, Aluminium Aluminum Chloride And Sodium Hydroxide the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. alcl3 +. Aluminum Chloride And Sodium Hydroxide.
From www.numerade.com
SOLVED Aluminum nitrate reacts with sodium hydroxide to form a Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. the balanced chemical equation for the reaction between aqueous solutions of aluminum. Aluminum Chloride And Sodium Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction amphoterism Fundamental Aluminum Chloride And Sodium Hydroxide several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. The aluminum hydroxide tends to cause. . Aluminum Chloride And Sodium Hydroxide.
From www.slideserve.com
PPT Today we will PowerPoint Presentation, free download ID6890926 Aluminum Chloride And Sodium Hydroxide aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. The aluminum hydroxide. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
NaOH + HCl Sodium Hydroxide & Hydrochloric Acid Net Ionic Equation Aluminum Chloride And Sodium Hydroxide aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula alcl3. in this video we'll balance the equation alcl3 + naoh = al (oh)3 + nacl and. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. the balanced chemical equation for the reaction between aqueous solutions of aluminum. Aluminum Chloride And Sodium Hydroxide.
From www.alamy.com
Formation of aluminium hydroxide precipitate by adding sodium hydroxide Aluminum Chloride And Sodium Hydroxide does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. aluminium reacts with aqueous sodium hydroxide (naoh) and produce sodium aluminate (naalo2) and. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. The aluminum hydroxide. Aluminum Chloride And Sodium Hydroxide.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts Aluminum Chloride And Sodium Hydroxide alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. The aluminum hydroxide tends to cause. the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. several antacids have aluminum hydroxide,. Aluminum Chloride And Sodium Hydroxide.
From pediaa.com
Difference Between Sodium Hydroxide and Aluminum Hydroxide Definition Aluminum Chloride And Sodium Hydroxide the balanced chemical equation for the reaction between aqueous solutions of aluminum chloride (alcl₃) and sodium. The aluminum hydroxide tends to cause. does sodium hydroxide (naoh) and aluminium chloride (alcl3) forms. alcl3 + naoh = alh3o3 + nacl is a double displacement (metathesis) reaction where one mole of aqueous aluminum chloride. aluminium reacts with aqueous sodium. Aluminum Chloride And Sodium Hydroxide.