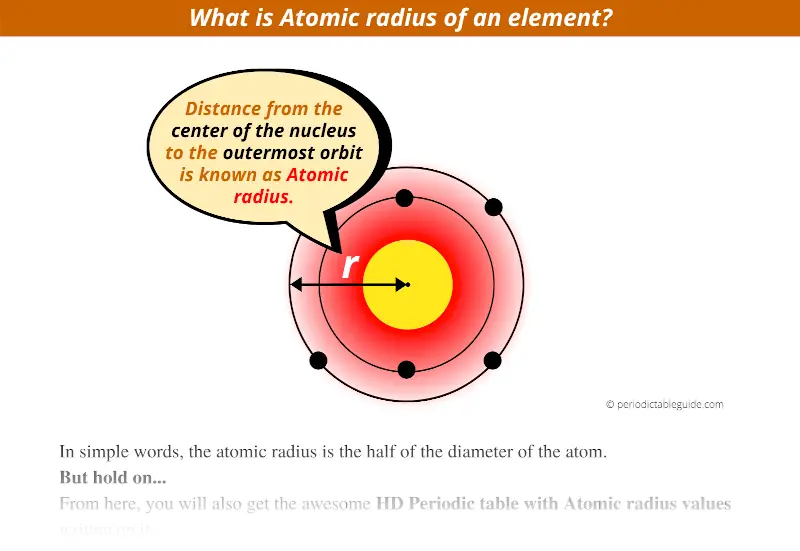
Smallest Atomic Radius To Largest Atomic Radius . Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Oxygen (o) is the only element in the list with a smaller atomic radius than s. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Which of these elements has a smaller atomic radius than sulfur (s): Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. 119 rows atomic radius of all the elements are mentioned in the chart below. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Below mentioned radii are the van der waals.
from periodictableguide.com
As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Which of these elements has a smaller atomic radius than sulfur (s): Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Oxygen (o) is the only element in the list with a smaller atomic radius than s. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Below mentioned radii are the van der waals. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2.
Get the Periodic table with Atomic radius values (Img+Chart)
Smallest Atomic Radius To Largest Atomic Radius Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Oxygen (o) is the only element in the list with a smaller atomic radius than s. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. 119 rows atomic radius of all the elements are mentioned in the chart below. As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. Below mentioned radii are the van der waals. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Which of these elements has a smaller atomic radius than sulfur (s):
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Below mentioned radii are the van der waals. Which of these elements has a smaller atomic radius than sulfur (s): As shown in the graph below, the atomic radius is largest at the first element in each. Smallest Atomic Radius To Largest Atomic Radius.
From www.youtube.com
Determining smallest atomic radius YouTube Smallest Atomic Radius To Largest Atomic Radius Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Which of these elements has a smaller atomic radius than sulfur. Smallest Atomic Radius To Largest Atomic Radius.
From worksheetlistjerry.z13.web.core.windows.net
Atomic Radius Worksheets Smallest Atomic Radius To Largest Atomic Radius Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. Which of these elements has a smaller atomic radius than sulfur (s): Below mentioned radii are the van der waals. The atomic radius of a chemical element is a measure of the size of its atom, usually, the. Smallest Atomic Radius To Largest Atomic Radius.
From www.youtube.com
Atomic Radius Bailey Parker YouTube Smallest Atomic Radius To Largest Atomic Radius Oxygen (o) is the only element in the list with a smaller atomic radius than s. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Which of these elements has a smaller atomic radius than sulfur (s): As shown in the graph below, the. Smallest Atomic Radius To Largest Atomic Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). 119 rows atomic radius of all the elements are mentioned in the chart below. Oxygen (o) is the only element in. Smallest Atomic Radius To Largest Atomic Radius.
From sciencenotes.org
Atomic Radius and Ionic Radius Smallest Atomic Radius To Largest Atomic Radius 119 rows atomic radius of all the elements are mentioned in the chart below. Oxygen (o) is the only element in the list with a smaller atomic radius than s. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. In the periodic table, atomic radii. Smallest Atomic Radius To Largest Atomic Radius.
From www.numerade.com
SOLVEDName and give the symbol of the element with the given Smallest Atomic Radius To Largest Atomic Radius Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. Which of these elements has a smaller atomic radius than sulfur (s): As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. The atomic. Smallest Atomic Radius To Largest Atomic Radius.
From study.com
What is Atomic Radius? Atomic Radius Examples & Periodic Trend Smallest Atomic Radius To Largest Atomic Radius As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. Below mentioned radii are the van der waals. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. 119 rows the atomic radius of. Smallest Atomic Radius To Largest Atomic Radius.
From www.wizeprep.com
Atomic Radius Trend Wize University Chemistry Textbook Wizeprep Smallest Atomic Radius To Largest Atomic Radius As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. 119 rows atomic radius of all the elements are mentioned in the. Smallest Atomic Radius To Largest Atomic Radius.
From elchoroukhost.net
Periodic Table Largest Atomic Radius Elcho Table Smallest Atomic Radius To Largest Atomic Radius As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. Which of these elements has a smaller atomic radius than sulfur (s):. Smallest Atomic Radius To Largest Atomic Radius.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Smallest Atomic Radius To Largest Atomic Radius Oxygen (o) is the only element in the list with a smaller atomic radius than s. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a. Smallest Atomic Radius To Largest Atomic Radius.
From freyabartlett.z13.web.core.windows.net
Size Of Atoms Chart Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. Which of these elements has a smaller atomic radius than sulfur (s): Periodic. Smallest Atomic Radius To Largest Atomic Radius.
From utedzz.blogspot.com
Periodic Table Smallest To Largest Radius Periodic Table Timeline Smallest Atomic Radius To Largest Atomic Radius Below mentioned radii are the van der waals. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). The atomic radius of. Smallest Atomic Radius To Largest Atomic Radius.
From 88guru.com
Atomic RadiusAn Overview 88Guru Smallest Atomic Radius To Largest Atomic Radius The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. 119 rows atomic radius of all the elements are mentioned in the chart below. Below mentioned radii are the van der waals. Atomic radii are measured in picometers (one picometer. Smallest Atomic Radius To Largest Atomic Radius.
From www.chegg.com
Solved Rank the following elements by atomic radius. Rank Smallest Atomic Radius To Largest Atomic Radius Oxygen (o) is the only element in the list with a smaller atomic radius than s. Below mentioned radii are the van der waals. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Which of these elements has a. Smallest Atomic Radius To Largest Atomic Radius.
From www.slideshare.net
Periodic trends Smallest Atomic Radius To Largest Atomic Radius As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. Below mentioned radii are the van der waals. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost. Smallest Atomic Radius To Largest Atomic Radius.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2432083 Smallest Atomic Radius To Largest Atomic Radius As shown in the graph below, the atomic radius is largest at the first element in each period, and it decreases down each period. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Hydrogen (h) has the smallest average. Smallest Atomic Radius To Largest Atomic Radius.
From bradleyrahman.z13.web.core.windows.net
Chart Of Atomic Radius Of Elements Smallest Atomic Radius To Largest Atomic Radius Oxygen (o) is the only element in the list with a smaller atomic radius than s. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center of the nucleus to the outermost isolated electron. Periodic trends indicate that atomic radius increases down a group (from top to bottom). Smallest Atomic Radius To Largest Atomic Radius.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Smallest Atomic Radius To Largest Atomic Radius Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. 119 rows the atomic radius of a chemical element is the distance from. Smallest Atomic Radius To Largest Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Smallest Atomic Radius To Largest Atomic Radius Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Which of these elements has a smaller atomic radius than sulfur (s):. Smallest Atomic Radius To Largest Atomic Radius.
From mac.ourora.co
Atomic Size Periodic Table Smallest Atomic Radius To Largest Atomic Radius Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest. Smallest Atomic Radius To Largest Atomic Radius.
From mindsstorm.weebly.com
Atomic radius chart mindsstorm Smallest Atomic Radius To Largest Atomic Radius Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. Below mentioned radii are the van der waals. Oxygen (o) is the only element in the list with a smaller atomic radius than s. Which of these elements has a smaller atomic radius than sulfur (s): In the. Smallest Atomic Radius To Largest Atomic Radius.
From www.breakingatom.com
Atomic Radius of Elements Smallest Atomic Radius To Largest Atomic Radius Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the center. Smallest Atomic Radius To Largest Atomic Radius.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Smallest Atomic Radius To Largest Atomic Radius Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. Oxygen (o) is the only element in the list with a smaller atomic radius than s. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell. Smallest Atomic Radius To Largest Atomic Radius.
From joialfrnq.blob.core.windows.net
Smallest Atomic Radius On Periodic Table at Juan McNair blog Smallest Atomic Radius To Largest Atomic Radius Below mentioned radii are the van der waals. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius. Smallest Atomic Radius To Largest Atomic Radius.
From fleetlokasin.weebly.com
Smallest to largest atomic radius fleetlokasin Smallest Atomic Radius To Largest Atomic Radius Which of these elements has a smaller atomic radius than sulfur (s): 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Oxygen (o) is the only element in the list with a smaller atomic radius than s. Because of these two trends, the largest. Smallest Atomic Radius To Largest Atomic Radius.
From www.coursehero.com
[Solved] 1.Rank the following elements by atomic radius. Rank from Smallest Atomic Radius To Largest Atomic Radius Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. The atomic radius of a chemical element is a measure of the size of its atom, usually, the distance from the. Smallest Atomic Radius To Largest Atomic Radius.
From www.crystalmaker.com
Elements, Atomic Radii and the Periodic Radii Smallest Atomic Radius To Largest Atomic Radius 119 rows atomic radius of all the elements are mentioned in the chart below. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down. Smallest Atomic Radius To Largest Atomic Radius.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Below mentioned radii are the van der waals. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. As shown in the graph below, the. Smallest Atomic Radius To Largest Atomic Radius.
From www.chem.fsu.edu
Electron Configurations Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atomic radii are measured in picometers (one picometer is equal to one. Smallest Atomic Radius To Largest Atomic Radius.
From socratic.org
For a given Period, which is the smallest atom? Socratic Smallest Atomic Radius To Largest Atomic Radius Which of these elements has a smaller atomic radius than sulfur (s): Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Below. Smallest Atomic Radius To Largest Atomic Radius.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Smallest Atomic Radius To Largest Atomic Radius Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. Periodic trends indicate that atomic radius increases down a group (from top to bottom) and from left to right across a period. 119 rows the atomic radius of. Smallest Atomic Radius To Largest Atomic Radius.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Smallest Atomic Radius To Largest Atomic Radius In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. As shown in the graph. Smallest Atomic Radius To Largest Atomic Radius.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy Smallest Atomic Radius To Largest Atomic Radius Oxygen (o) is the only element in the list with a smaller atomic radius than s. Below mentioned radii are the van der waals. In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Which of these elements has a smaller atomic radius than sulfur (s): As. Smallest Atomic Radius To Largest Atomic Radius.
From mungfali.com
Atomic Radius Chart Periodic Table Smallest Atomic Radius To Largest Atomic Radius Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure 8.2.4 8.2. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The atomic radius. Smallest Atomic Radius To Largest Atomic Radius.