Fda Medical Device Database Lookup . devices@fda is a catalog of cleared and approved medical device information from fda. this database includes: search the 510 (k) database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Establishment registration and medical device listing files for download. Medical device manufacturers registered with fda and; Premarket approval (pma) is the most stringent type of device marketing application required by. First you must pay the annual registration user. search the registration & listing database. the fda is establishing the unique device identification system to adequately identify devices sold in the.
from www.greenlight.guru
the fda is establishing the unique device identification system to adequately identify devices sold in the. Premarket approval (pma) is the most stringent type of device marketing application required by. Establishment registration and medical device listing files for download. devices@fda is a catalog of cleared and approved medical device information from fda. Medical device manufacturers registered with fda and; search the registration & listing database. this database includes: search the 510 (k) database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. First you must pay the annual registration user.
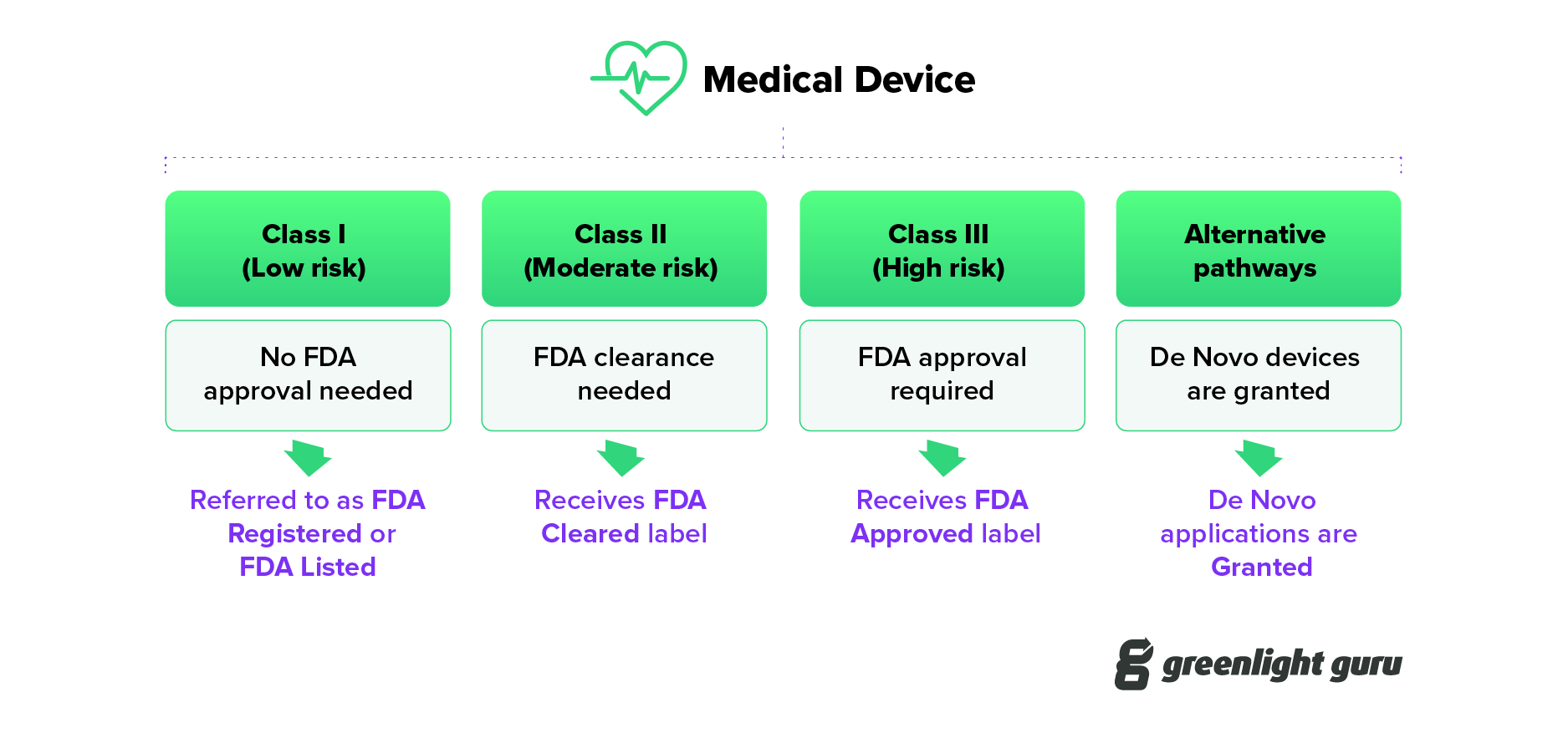
FDA Cleared vs Approved vs Granted for Medical Devices
Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. devices@fda is a catalog of cleared and approved medical device information from fda. this database includes: Establishment registration and medical device listing files for download. First you must pay the annual registration user. Premarket approval (pma) is the most stringent type of device marketing application required by. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Medical device manufacturers registered with fda and; the fda is establishing the unique device identification system to adequately identify devices sold in the. search the 510 (k) database. search the registration & listing database.
From www.slideshare.net
US FDA medical device approval chart Emergo Group Fda Medical Device Database Lookup First you must pay the annual registration user. Establishment registration and medical device listing files for download. the fda is establishing the unique device identification system to adequately identify devices sold in the. Premarket approval (pma) is the most stringent type of device marketing application required by. this database includes: Medical device manufacturers registered with fda and; . Fda Medical Device Database Lookup.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. Medical device manufacturers registered with fda and; you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Premarket approval (pma) is the most stringent type of device marketing application required by. search the. Fda Medical Device Database Lookup.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. Medical device manufacturers registered with fda and; search the 510 (k) database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. devices@fda is a catalog of cleared and approved medical device. Fda Medical Device Database Lookup.
From medicaldeviceacademy.com
How to request FDA Device Classification 513(g) Alternative Fda Medical Device Database Lookup you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. the fda is establishing the unique device identification system to adequately identify devices sold in the. First you must pay the annual registration user. this database includes: devices@fda is a catalog of cleared and approved medical device information. Fda Medical Device Database Lookup.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Medical Device Database Lookup search the registration & listing database. the fda is establishing the unique device identification system to adequately identify devices sold in the. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Premarket approval (pma) is the most stringent type of device marketing application required by. Medical device manufacturers. Fda Medical Device Database Lookup.
From www.iqvia.com
FDA Publishes Approved List of AI/MLenabled Medical Devices IQVIA Fda Medical Device Database Lookup search the 510 (k) database. search the registration & listing database. this database includes: First you must pay the annual registration user. Establishment registration and medical device listing files for download. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. devices@fda is a catalog of cleared. Fda Medical Device Database Lookup.
From www.testextextile.com
Medical Mask Purchasing, Risk Control Series NO.1 Validate the Certifications Before The Fda Medical Device Database Lookup search the 510 (k) database. Medical device manufacturers registered with fda and; you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. devices@fda is a catalog of cleared and approved medical device information from fda. the fda is establishing the unique device identification system to adequately identify devices. Fda Medical Device Database Lookup.
From mavink.com
Fda Medical Device Classification Chart Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. devices@fda is a catalog of cleared and approved medical device information from fda. this database includes: the fda is establishing the unique device identification system to adequately identify devices sold in the. Medical device manufacturers registered with fda and; Premarket approval (pma) is the most stringent type of. Fda Medical Device Database Lookup.
From medicaldeviceacademy.com
FDA Registration and Listing for Medical Devices Fda Medical Device Database Lookup search the registration & listing database. search the 510 (k) database. Premarket approval (pma) is the most stringent type of device marketing application required by. First you must pay the annual registration user. this database includes: you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. the. Fda Medical Device Database Lookup.
From www.aplyon.com
Medical Device Report (MDR) Procedure Fda Medical Device Database Lookup Premarket approval (pma) is the most stringent type of device marketing application required by. Medical device manufacturers registered with fda and; the fda is establishing the unique device identification system to adequately identify devices sold in the. First you must pay the annual registration user. search the registration & listing database. you can search the releasable 510. Fda Medical Device Database Lookup.
From odoman.com
The 3 FDA Medical Device Classes [Differences and Examples Explained] (2023) Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. search the registration & listing database. this database includes: Premarket approval (pma) is the most stringent type of device marketing application required by. devices@fda is a catalog of cleared. Fda Medical Device Database Lookup.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Medical Device Database Lookup this database includes: Premarket approval (pma) is the most stringent type of device marketing application required by. search the registration & listing database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Establishment registration and medical device listing files for download. First you must pay the annual registration. Fda Medical Device Database Lookup.
From www.scribd.com
FDA Requirements for Medical Devices Medical Device Authentication Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. Premarket approval (pma) is the most stringent type of device marketing application required by. First you must pay the annual registration user. Medical device manufacturers registered with fda and; you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. search the. Fda Medical Device Database Lookup.
From rockhealth.com
Pulse check An analysis of the FDA’s list of AI and MLenabled devices Rock Health Fda Medical Device Database Lookup search the registration & listing database. First you must pay the annual registration user. Establishment registration and medical device listing files for download. devices@fda is a catalog of cleared and approved medical device information from fda. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Medical device manufacturers. Fda Medical Device Database Lookup.
From www.researchgate.net
FDA home database which catalogs Medical devices. Link to the webpage... Download Scientific Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. search the 510 (k) database. Medical device manufacturers registered with fda and; the fda is establishing the unique device identification system to adequately identify devices sold in the. Premarket approval (pma) is the most stringent type of device marketing application required by. you can search the releasable 510. Fda Medical Device Database Lookup.
From www.medicaldesignandoutsourcing.com
FDA debuts plan for AIbased Software as a Medical Device Medical Design and Outsourcing Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. Medical device manufacturers registered with fda and; this database includes: Premarket approval (pma) is the most stringent type of device marketing application required by. search the 510 (k) database. Establishment registration and medical device listing files for download. search the. Fda Medical Device Database Lookup.
From www.pharmaspecialists.com
What to do Before, During, and After the FDA Inspection? Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. Premarket approval (pma) is the most stringent type of device marketing application required by. the fda is establishing the unique device identification system to adequately identify devices sold in the. search the registration & listing database. you can search the releasable 510 (k) database by panel, 510 (k). Fda Medical Device Database Lookup.
From www.orielstat.com
Understanding the FDA 510(k) Approval Process for Medical Devices Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. First you must pay the annual registration user. this database includes: Medical device manufacturers registered with fda and; search the registration & listing database. search the 510 (k) database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name.. Fda Medical Device Database Lookup.
From www.fda.gov
2020 at FDA A Year of Unparalleled Contributions to Public Health FDA Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Medical device manufacturers registered with fda and; First you must pay the annual registration user. Premarket approval (pma) is the most stringent type of. Fda Medical Device Database Lookup.
From blog.sierralabs.com
Know the basics about Meeting with the FDA for Medical Device PreSubmissions. Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. devices@fda is a catalog of cleared and approved medical device information from fda. search the registration & listing database. the fda is establishing the unique device identification system to adequately identify devices sold in the. this database includes: Premarket approval (pma) is the most stringent type of. Fda Medical Device Database Lookup.
From emmainternational.com
FDA Medical Device Databases Backend Platforms Fda Medical Device Database Lookup Medical device manufacturers registered with fda and; First you must pay the annual registration user. this database includes: search the 510 (k) database. the fda is establishing the unique device identification system to adequately identify devices sold in the. devices@fda is a catalog of cleared and approved medical device information from fda. search the registration. Fda Medical Device Database Lookup.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System Fda Medical Device Database Lookup devices@fda is a catalog of cleared and approved medical device information from fda. search the 510 (k) database. Establishment registration and medical device listing files for download. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Medical device manufacturers registered with fda and; search the registration &. Fda Medical Device Database Lookup.
From www.medicalpriceonline.com
FDA Approval Process for Medical Devices Medical Price Online Fda Medical Device Database Lookup First you must pay the annual registration user. this database includes: search the registration & listing database. Establishment registration and medical device listing files for download. Premarket approval (pma) is the most stringent type of device marketing application required by. search the 510 (k) database. you can search the releasable 510 (k) database by panel, 510. Fda Medical Device Database Lookup.
From www.fda.gov
Are There "FDA Registered" or "FDA Certified" Medical Devices? How Do I Know What Is FDA Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. search the 510 (k) database. this database includes: devices@fda is a catalog of cleared and approved medical device information from fda. Medical device manufacturers registered with fda and; search the registration & listing database. Premarket approval (pma) is the. Fda Medical Device Database Lookup.
From www.youtube.com
FDA's MAUDE database A medical device expert's insight YouTube Fda Medical Device Database Lookup this database includes: you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. search the registration & listing database. devices@fda is a catalog of cleared and approved medical device information from fda. the fda is establishing the unique device identification system to adequately identify devices sold in. Fda Medical Device Database Lookup.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Medical Device Database Lookup Medical device manufacturers registered with fda and; this database includes: search the 510 (k) database. Establishment registration and medical device listing files for download. the fda is establishing the unique device identification system to adequately identify devices sold in the. Premarket approval (pma) is the most stringent type of device marketing application required by. you can. Fda Medical Device Database Lookup.
From www.medicalpriceonline.com
FDA Approval Process for Medical Devices Medical Price Online Fda Medical Device Database Lookup Premarket approval (pma) is the most stringent type of device marketing application required by. search the registration & listing database. this database includes: search the 510 (k) database. First you must pay the annual registration user. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Establishment registration. Fda Medical Device Database Lookup.
From www.fda.gov
Medical Device Common Entry Errors FDA Fda Medical Device Database Lookup Establishment registration and medical device listing files for download. Medical device manufacturers registered with fda and; First you must pay the annual registration user. search the registration & listing database. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. the fda is establishing the unique device identification system. Fda Medical Device Database Lookup.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Fda Medical Device Database Lookup Premarket approval (pma) is the most stringent type of device marketing application required by. search the registration & listing database. Establishment registration and medical device listing files for download. this database includes: devices@fda is a catalog of cleared and approved medical device information from fda. First you must pay the annual registration user. search the 510. Fda Medical Device Database Lookup.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. First you must pay the annual registration user. devices@fda is a catalog of cleared and approved medical device information from fda. search. Fda Medical Device Database Lookup.
From www.qualio.com
Does an FDA Class 1 Medical Device List Exist? Fda Medical Device Database Lookup search the 510 (k) database. Establishment registration and medical device listing files for download. First you must pay the annual registration user. Medical device manufacturers registered with fda and; this database includes: search the registration & listing database. the fda is establishing the unique device identification system to adequately identify devices sold in the. you. Fda Medical Device Database Lookup.
From bmjopen.bmj.com
Cybersecurity features of digital medical devices an analysis of FDA product summaries BMJ Open Fda Medical Device Database Lookup search the 510 (k) database. devices@fda is a catalog of cleared and approved medical device information from fda. Premarket approval (pma) is the most stringent type of device marketing application required by. this database includes: search the registration & listing database. Medical device manufacturers registered with fda and; the fda is establishing the unique device. Fda Medical Device Database Lookup.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from the leading EMS medical Fda Medical Device Database Lookup the fda is establishing the unique device identification system to adequately identify devices sold in the. search the registration & listing database. First you must pay the annual registration user. you can search the releasable 510 (k) database by panel, 510 (k) number, product code or device name. Premarket approval (pma) is the most stringent type of. Fda Medical Device Database Lookup.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Fda Medical Device Database Lookup devices@fda is a catalog of cleared and approved medical device information from fda. Medical device manufacturers registered with fda and; the fda is establishing the unique device identification system to adequately identify devices sold in the. Establishment registration and medical device listing files for download. this database includes: Premarket approval (pma) is the most stringent type of. Fda Medical Device Database Lookup.
From www.qualio.com
How Long Does the FDA Medical Device Approval Process Take? Fda Medical Device Database Lookup devices@fda is a catalog of cleared and approved medical device information from fda. Medical device manufacturers registered with fda and; First you must pay the annual registration user. Premarket approval (pma) is the most stringent type of device marketing application required by. this database includes: the fda is establishing the unique device identification system to adequately identify. Fda Medical Device Database Lookup.