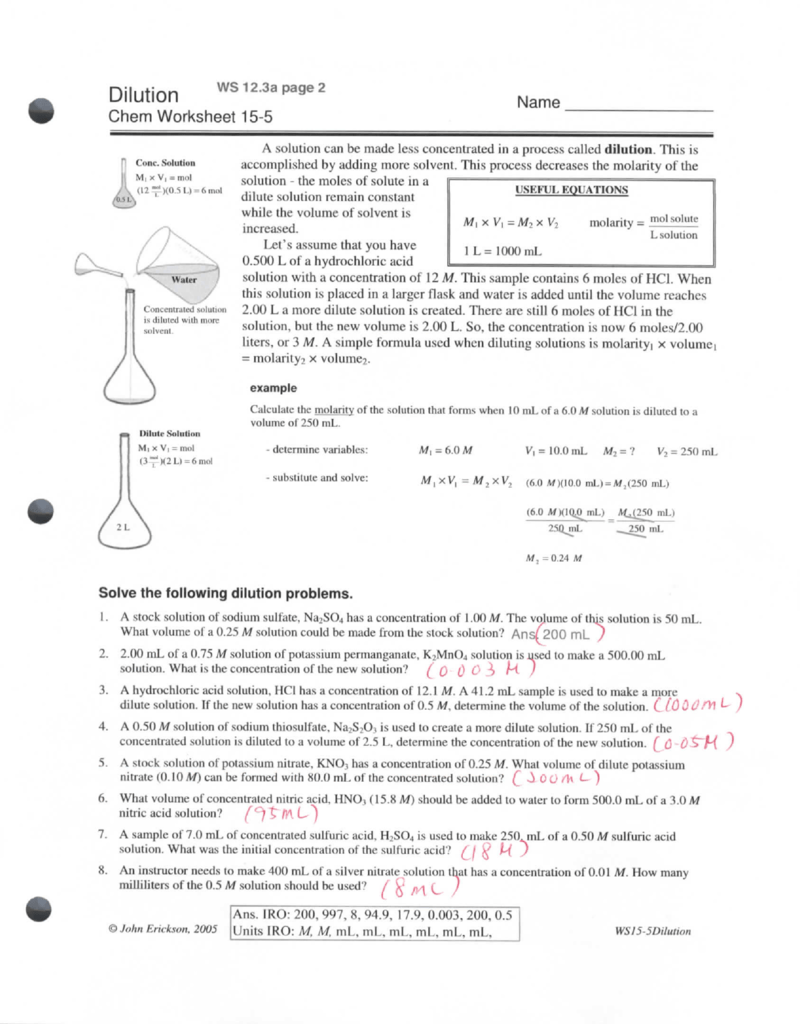
Chemistry Dilutions Worksheet Answers . For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1200 ml will be the final volume of the solution. How much of it do you need to prepare 50 ml of a. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. Perform the following dilutions calculation. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water.
from studylib.net
Perform the following dilutions calculation. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. 1200 ml will be the final volume of the solution. Dilution m 1v 1=m 2v 2 1. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. There’s a bottle of 0.750 m nacl on a shelf.
Dilution
Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. 1200 ml will be the final volume of the solution. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. There’s a bottle of 0.750 m nacl on a shelf. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water.
From studylib.net
Dilution Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. 1200 ml will be the final volume of the solution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the.. Chemistry Dilutions Worksheet Answers.
From thekidsworksheet.com
Serial Dilutions Worksheet Answers Thekidsworksheet Chemistry Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. However, since. Chemistry Dilutions Worksheet Answers.
From thekidsworksheet.com
Dilutions Worksheet Answer Key Thekidsworksheet Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. There’s a bottle of 0.750 m nacl on a shelf. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 2) if. Chemistry Dilutions Worksheet Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Chemistry Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. Perform the following dilutions calculation. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. 1200 ml will be the final volume of the solution. How much of it do you need to prepare 50 ml of a. 2) if i dilute. Chemistry Dilutions Worksheet Answers.
From lessoncampusunspelt.z13.web.core.windows.net
Molarity Worksheet 1 Answer Key Chemistry Chemistry Dilutions Worksheet Answers How much of it do you need to prepare 50 ml of a. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. 1200 ml will be. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Chemistry Dilutions Worksheet Answers How much of it do you need to prepare 50 ml of a. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 1) if i add 25 ml of. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilutions Worksheet essentials.pdf Name Part II Dilutions Chemistry Dilutions Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. There’s a bottle of 0.750 m nacl. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Dilution Calculation Worksheet With Answers Printable Pdf Download Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. 1200 ml will be the final volume of the solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m. Chemistry Dilutions Worksheet Answers.
From www.scribd.com
Dilutions Worksheet Key For Chemistry PDF Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a. Chemistry Dilutions Worksheet Answers.
From materialmagicsusana.z19.web.core.windows.net
Dilutions Worksheets Answers Chemistry Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. How much of it do you need to prepare 50 ml of a. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Molarity and Dilutions Notes and Worksheet Set by Chemistry Wiz Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Solved Dilution Worksheet 2 Show all work in the spaces Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Subatomic Particles And Isotopes Worksheets Answers Chemistry Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. 1200 ml will be the final volume of the solution. 1) if i add 25 ml of. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Chemistry 12 Worksheet 42 Bronsted Acids And Equilibrium Answers Chemistry Dilutions Worksheet Answers However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration. Chemistry Dilutions Worksheet Answers.
From studylib.net
Solutions & Dilutions Worksheet Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Molarity and Dilutions Notes and Worksheet Set by Chemistry Wiz Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because. Chemistry Dilutions Worksheet Answers.
From davida.davivienda.com
Molarity And Dilution Worksheet Answers Chemistry Printable Word Searches Chemistry Dilutions Worksheet Answers Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. There’s a bottle of 0.750 m nacl on a shelf. Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. If you dilute 175 ml. Chemistry Dilutions Worksheet Answers.
From worksheets.decoomo.com
20++ Dilutions Worksheet Answers Worksheets Decoomo Chemistry Dilutions Worksheet Answers If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. Chemistry Dilutions Worksheet Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Chemistry Dilutions Worksheet Answers If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. However, since there’s already 500 ml of solution present, you only. Chemistry Dilutions Worksheet Answers.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water.. Chemistry Dilutions Worksheet Answers.
From pic-cohn.blogspot.com
Dilution Worksheet Chemistry Serial Dilutions Worksheet With Answer Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. 1200 ml will be the final volume of the solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. There’s a bottle of 0.750 m nacl on a shelf. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Scps Chemistry Worksheet With Answers Periodicity Printable Pdf Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. Perform. Chemistry Dilutions Worksheet Answers.
From quizzlistreplevies.z13.web.core.windows.net
Molarity And Dilution Worksheet Answers Chemistry Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 2) if i dilute 250 ml of 0.10 m. Chemistry Dilutions Worksheet Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. For questions 1 and 2, the units for your final answer should be. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Chemistry Dilutions Worksheet Answers If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 1200 ml will be the final volume of the solution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. There’s a bottle. Chemistry Dilutions Worksheet Answers.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Chemistry Dilutions Worksheet Answers Perform the following dilutions calculation. Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. 1200 ml will be the final volume of the solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 2) if i. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. How much of it do you need to prepare 50 ml of a. If you dilute 175 ml of a 1.6 m. Chemistry Dilutions Worksheet Answers.
From davida.davivienda.com
Molarity And Dilution Worksheet Answers Chemistry Printable Word Searches Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of. Chemistry Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Predicting The Products Of Chemical Reactions Chemistry Worksheet Chemistry Dilutions Worksheet Answers 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1) if i add 25 ml of. Chemistry Dilutions Worksheet Answers.
From lessondbjack.z13.web.core.windows.net
Dilutions Worksheet Chemistry Answers Chemistry Dilutions Worksheet Answers However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. How much of it do you need to prepare 50 ml of a. Perform the following dilutions calculation. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this. Chemistry Dilutions Worksheet Answers.
From www.studocu.com
Grade 11 Chemistry Dilution Worksheet © John Erickson, 2005 WS15 Chemistry Dilutions Worksheet Answers 1200 ml will be the final volume of the solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume of 750 ml, what will the concentration of this solution. Perform the following. Chemistry Dilutions Worksheet Answers.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Chemistry Dilutions Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Dilution m 1v 1=m 2v 2 1. 1200 ml will be the final volume of the solution. 2) if i dilute 250 ml of 0.10 m lithium acetate solution to a volume. Chemistry Dilutions Worksheet Answers.
From studylib.net
Dilutions Worksheet 11UChem Chemistry Dilutions Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1200 ml will be the final volume of the solution. How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to. Chemistry Dilutions Worksheet Answers.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Chemistry Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1200 ml will be the final volume of the solution. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. If you dilute 175 ml of a 1.6 m solution of licl. Chemistry Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilution SelfStudy Worksheet Dilutions 1 Spring 2020 Dilutions Chemistry Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. Perform the following dilutions calculation. 1200 ml will be the final volume of the solution. Dilution m 1v 1=m 2v 2 1. However, since there’s already 500 ml of solution present, you only need to add 700 ml of water. 1) if i add 25 ml of water to 125. Chemistry Dilutions Worksheet Answers.