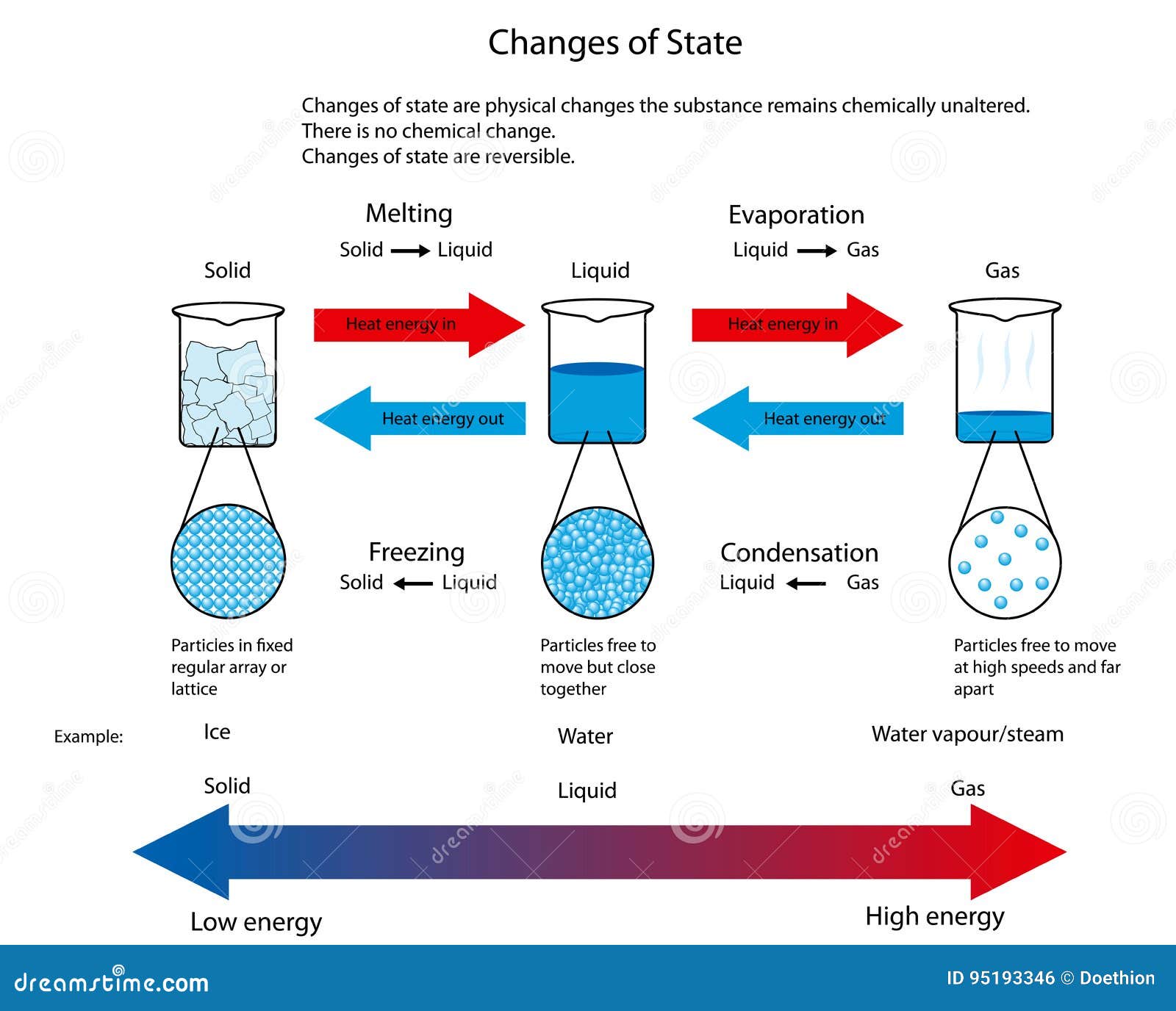
Solid To.liquid . The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solids can melt into liquids or sublime into gases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Solids form by deposition from gases or freezing of liquids. The conversion of a solid to a liquid is called fusion (or melting). Changing states of matter occur when matter loses or absorbs energy. Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form?
from www.dreamstime.com
Changing states of matter occur when matter loses or absorbs energy. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids can melt into liquids or sublime into gases. The conversion of a solid to a liquid is called fusion (or melting). We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Solids form by deposition from gases or freezing of liquids. You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form?
Illustration for Changes of State between Solid, Liquid and Gas Stock
Solid To.liquid You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids form by deposition from gases or freezing of liquids. Changing states of matter occur when matter loses or absorbs energy. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids can melt into liquids or sublime into gases. The conversion of a solid to a liquid is called fusion (or melting).
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid To.liquid The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Changing. Solid To.liquid.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Solid To.liquid Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids form by deposition from gases or freezing of liquids. The conversion of a solid to a liquid is called fusion (or melting). Solids can melt into liquids or sublime into gases. You would have observed changing states of matter when ice cubes. Solid To.liquid.
From www.ase.org.uk
Solids, liquids and gases Solid To.liquid Solids can melt into liquids or sublime into gases. Changing states of matter occur when matter loses or absorbs energy. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. The conversion of a solid to. Solid To.liquid.
From ar.inspiredpencil.com
Examples Of Liquids Solid To.liquid You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). We take advantage of changes between the gas, liquid, and solid states. Solid To.liquid.
From ar.inspiredpencil.com
Solid Particles Animation Solid To.liquid You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids form by deposition from gases or freezing of liquids. The energy. Solid To.liquid.
From www.vrogue.co
Solids Liquids Gases vrogue.co Solid To.liquid Changing states of matter occur when matter loses or absorbs energy. Solids can melt into liquids or sublime into gases. Solids form by deposition from gases or freezing of liquids. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas),. Solid To.liquid.
From ar.inspiredpencil.com
Solids Liquids And Gases Worksheets Solid To.liquid Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). The. Solid To.liquid.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids can melt into liquids or sublime into gases. The energy required to melt 1 mol. Solid To.liquid.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Solid To.liquid Solids form by deposition from gases or freezing of liquids. Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). We take advantage of changes between the gas, liquid, and solid states to cool a drink. Solid To.liquid.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? We take advantage of changes between the gas, liquid, and solid states to cool a drink. Solid To.liquid.
From sciencenotes.org
Liquid Definition Examples of Liquids Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). Solids form by deposition from gases or freezing of liquids. You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Explore the interactive simulation of matter. Solid To.liquid.
From www.britannica.com
phase Definition & Facts Britannica Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solids can melt into liquids or sublime into gases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to. Solid To.liquid.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid To.liquid Changing states of matter occur when matter loses or absorbs energy. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Explore the interactive simulation of matter states. Solid To.liquid.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Solid To.liquid Solids form by deposition from gases or freezing of liquids. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solids can melt into liquids or sublime into gases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our. Solid To.liquid.
From mainanspektakuler.blogspot.com
25+ Gambar Mencair Solid To.liquid Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). You would have observed changing states of matter when ice cubes melt from. Solid To.liquid.
From www.studocu.com
Science GASES, LIQUIDS, AND SOLIDS Atoms, molecules, and/or ions are Solid To.liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Changing. Solid To.liquid.
From www.snexplores.org
Explainer What are the different states of matter? Solid To.liquid You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids form by deposition from gases or freezing of liquids. Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. We take. Solid To.liquid.
From worksheetlistutu.z13.web.core.windows.net
Liquids And Solids Worksheet Solid To.liquid You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids form by deposition from gases or freezing of liquids. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1. Solid To.liquid.
From www.dreamstime.com
Density and States of Matter Stock Vector Illustration of science Solid To.liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Solids form by deposition from gases or freezing of liquids. Changing states of matter occur when matter loses. Solid To.liquid.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion Solid To.liquid The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by. Solid To.liquid.
From www.geeksforgeeks.org
Difference Between Solid, Liquid, and Gas In Tabular Form Solid To.liquid The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids form by deposition from gases or freezing of liquids. Explore the. Solid To.liquid.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To.liquid Solids form by deposition from gases or freezing of liquids. The conversion of a solid to a liquid is called fusion (or melting). We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas. Solid To.liquid.
From learningschoolboyer.z13.web.core.windows.net
Show The Phase Change Diagram Process Solid To.liquid Solids can melt into liquids or sublime into gases. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Solids form by deposition from gases or freezing of liquids. Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. We take advantage of changes between the. Solid To.liquid.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh. Solid To.liquid.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid To.liquid Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. Changing states of matter occur when matter loses or absorbs energy. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting).. Solid To.liquid.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Solid To.liquid Solids can melt into liquids or sublime into gases. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside. Solid To.liquid.
From www.askiitians.com
Classification of Solids Study Material for IIT JEE askIITians Solid To.liquid The conversion of a solid to a liquid is called fusion (or melting). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids can melt into liquids or sublime into gases. Explore the interactive simulation of matter states. Solid To.liquid.
From saylordotorg.github.io
Solids and Liquids Solid To.liquid Solids can melt into liquids or sublime into gases. Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Solids form by. Solid To.liquid.
From www.youtube.com
Solid in liquid solution YouTube Solid To.liquid Changing states of matter occur when matter loses or absorbs energy. Solids form by deposition from gases or freezing of liquids. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into. Solid To.liquid.
From sciencenotes.org
States of Matter Solid To.liquid Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids can melt into liquids or sublime into gases. The conversion of a solid to a liquid is called fusion (or melting). Solids form by deposition from gases or freezing of liquids. We take advantage of changes between the gas, liquid, and solid. Solid To.liquid.
From www.radixtree.com
Physics Matter Online Education System Solid To.liquid Solids form by deposition from gases or freezing of liquids. Solids can melt into liquids or sublime into gases. The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). The conversion of a solid to a liquid is called fusion (or melting). You would have observed changing states of matter when ice cubes. Solid To.liquid.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Solid To.liquid Solids form by deposition from gases or freezing of liquids. Changing states of matter occur when matter loses or absorbs energy. Solids can melt into liquids or sublime into gases. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas),. Solid To.liquid.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram Solid To.liquid Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids can melt into liquids or sublime into gases. The conversion of a solid to a liquid is called fusion (or melting). The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). We take advantage of. Solid To.liquid.
From www.tutorix.com
Matter exists in three physical forms solid liquid Tutorix Solid To.liquid The energy required to melt 1 mol of a substance is its enthalpy of fusion (δh fus). Explore the interactive simulation of matter states and learn about phase changes, temperature effects, and atomic interactions. Solids can melt into liquids or sublime into gases. The conversion of a solid to a liquid is called fusion (or melting). Solids form by deposition. Solid To.liquid.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid To.liquid Changing states of matter occur when matter loses or absorbs energy. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and cool food inside a refrigerator (gas to liquid and vice versa). Solids form by deposition from gases or. Solid To.liquid.