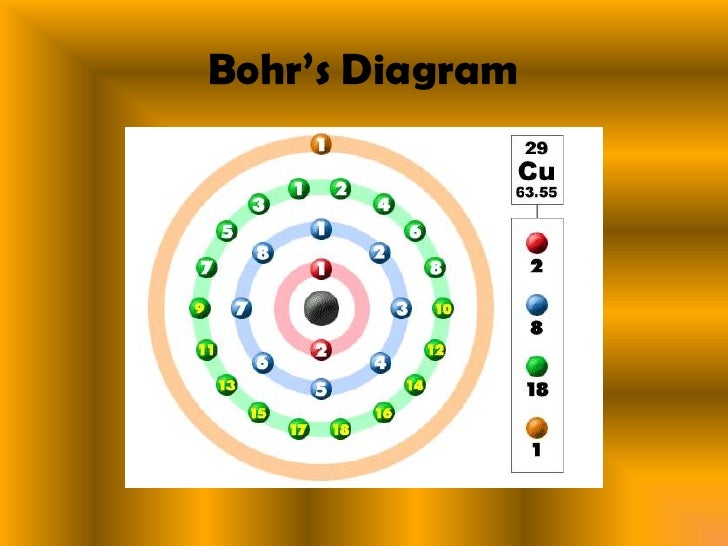
Copper Electron Configuration Orbital . Electron configuration describes the distribution of electrons within an atom’s orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. There are two valence electrons in the copper. What is the electron configuration of copper? Since 1s can only hold two electrons the next 2. Copper is in the ninth column of the transition metals in the d block of the fourth. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The full electron configuration for copper is [ar] 3d10 4s1. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9.
from www.slideshare.net
In writing the electron configuration for copper the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. There are two valence electrons in the copper. Since 1s can only hold two electrons the next 2. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The full electron configuration for copper is [ar] 3d10 4s1. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper is in the ninth column of the transition metals in the d block of the fourth. Electron configuration describes the distribution of electrons within an atom’s orbitals. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹.
Copper
Copper Electron Configuration Orbital Electron configuration describes the distribution of electrons within an atom’s orbitals. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Since 1s can only hold two electrons the next 2. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. The full electron configuration for copper is [ar] 3d10 4s1. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. What is the electron configuration of copper? There are two valence electrons in the copper. Copper is in the ninth column of the transition metals in the d block of the fourth. Electron configuration describes the distribution of electrons within an atom’s orbitals. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration Orbital The full electron configuration for copper is [ar] 3d10 4s1. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Copper is in the ninth column of the transition metals in the d block of the fourth. 1s 2 2s 2 2p 6. Copper Electron Configuration Orbital.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electron Configuration Orbital This means that the 29 electrons are distributed among the various orbitals available in the copper atom. Copper is in the ninth column of the transition metals in the d block of the fourth. There are two valence electrons in the copper. Electron configuration describes the distribution of electrons within an atom’s orbitals. The full electron configuration for copper is. Copper Electron Configuration Orbital.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Orbital Since 1s can only hold two electrons the next 2. There are two valence electrons in the copper. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. In. Copper Electron Configuration Orbital.
From fity.club
Orbital Diagram For Copper Copper Electron Configuration Orbital There are two valence electrons in the copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Since 1s can only hold two electrons the next 2. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. In its ground state, copper has the. Copper Electron Configuration Orbital.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Electron configuration describes the distribution of electrons within an atom’s orbitals. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. The full electron configuration for copper is [ar] 3d10 4s1. Since 1s can only hold. Copper Electron Configuration Orbital.
From fity.club
Orbital Diagram For Copper Copper Electron Configuration Orbital In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. Since 1s can only hold two electrons the next 2. What is the electron configuration of copper? The full electron configuration for. Copper Electron Configuration Orbital.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Orbital What is the electron configuration of copper? This means that the 29 electrons are distributed among the various orbitals available in the copper atom. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Electron configuration describes the distribution of electrons within an atom’s orbitals. 1s. Copper Electron Configuration Orbital.
From slideplayer.com
Electrons in Atoms Electron Configuration ppt download Copper Electron Configuration Orbital In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The full electron configuration for copper is [ar] 3d10 4s1. Copper is in the ninth column of the transition metals in the d block of the fourth. This means that the 29 electrons are distributed among the various orbitals available in the copper. Copper Electron Configuration Orbital.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Orbital 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. This means that the 29 electrons are distributed. Copper Electron Configuration Orbital.
From mungfali.com
Orbital Diagram Of Copper Copper Electron Configuration Orbital In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. Since 1s can only hold two electrons the next 2. What is the electron configuration of copper? This means that the 29 electrons are distributed among the various orbitals available in the copper atom. Electron configuration. Copper Electron Configuration Orbital.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Orbital There are two valence electrons in the copper. The full electron configuration for copper is [ar] 3d10 4s1. Copper is in the ninth column of the transition metals in the d block of the fourth. What is the electron configuration of copper? 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. This means that. Copper Electron Configuration Orbital.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Electron Configuration Orbital Electron configuration describes the distribution of electrons within an atom’s orbitals. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. What is the electron configuration of copper? There are two valence electrons in the copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. This means that the 29 electrons are. Copper Electron Configuration Orbital.
From fity.club
Orbital Diagram For Copper Copper Electron Configuration Orbital The full electron configuration for copper is [ar] 3d10 4s1. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. Electron configuration describes the distribution of electrons within an atom’s orbitals. What is the electron configuration. Copper Electron Configuration Orbital.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper Electron Configuration Orbital This means that the 29 electrons are distributed among the various orbitals available in the copper atom. What is the electron configuration of copper? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper is in the ninth column of the transition metals in the d block of the fourth. Copper’s electron. Copper Electron Configuration Orbital.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Orbital Electron configuration describes the distribution of electrons within an atom’s orbitals. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Since 1s can only hold two electrons the next 2. Copper is in the ninth column of the transition metals in the d block of. Copper Electron Configuration Orbital.
From schematicmaxeywheezle.z21.web.core.windows.net
Orbital Energy Diagram For Copper Copper Electron Configuration Orbital Copper is in the ninth column of the transition metals in the d block of the fourth. The full electron configuration for copper is [ar] 3d10 4s1. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. What is the. Copper Electron Configuration Orbital.
From fity.club
Electron Configuration Of Copper Copper Electron Configuration Orbital 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹.. Copper Electron Configuration Orbital.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Copper is in the ninth column of the transition metals in the d block of the fourth. Electron configuration describes the distribution of electrons within an atom’s orbitals. Since 1s can only hold two electrons the next 2. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. What is the electron configuration of copper? The full electron configuration for copper is [ar]. Copper Electron Configuration Orbital.
From kethewtdudley.blogspot.com
Electronic Configuration of Copper KethewtDudley Copper Electron Configuration Orbital 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Electron configuration describes the distribution of electrons within an atom’s orbitals. What is the electron configuration of copper? The full electron configuration for copper is [ar] 3d10 4s1. Since 1s can only hold two electrons the next 2. This means that the 29 electrons are distributed among. Copper Electron Configuration Orbital.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Orbital This means that the 29 electrons are distributed among the various orbitals available in the copper atom. Since 1s can only hold two electrons the next 2. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing. Copper Electron Configuration Orbital.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration Orbital Copper is in the ninth column of the transition metals in the d block of the fourth. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2. What is the electron configuration of copper? There are two valence electrons in the copper. The. Copper Electron Configuration Orbital.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Copper Electron Configuration Orbital Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. Copper is in the ninth column of the transition metals in the d block of the fourth. What is the electron configuration of copper? 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. In. Copper Electron Configuration Orbital.
From www.slideshare.net
Copper Copper Electron Configuration Orbital What is the electron configuration of copper? There are two valence electrons in the copper. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. Since 1s can only hold two electrons the next 2. Electron configuration describes the distribution of electrons within an atom’s orbitals. The electron distribution in copper spans across the k,. Copper Electron Configuration Orbital.
From fity.club
Electron Configuration Of Copper Copper Electron Configuration Orbital In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Since 1s can only hold two electrons the next 2. Copper is in the ninth column. Copper Electron Configuration Orbital.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration Orbital Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. What is the electron configuration of copper? 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in. Copper Electron Configuration Orbital.
From schemesnet.com
Orbital Diagram Copper Copper Electron Configuration Orbital 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. There are two valence electrons in the copper. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. Copper is in the ninth column of the transition metals in the d block of the fourth. 1s2 2s2 2p6. Copper Electron Configuration Orbital.
From mungfali.com
Orbital Diagram Of Copper Copper Electron Configuration Orbital In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. The full electron configuration for copper is [ar] 3d10 4s1. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. What is the electron configuration of copper? This means that the 29 electrons are distributed among the various orbitals. Copper Electron Configuration Orbital.
From fity.club
Orbital Diagram For Copper Copper Electron Configuration Orbital In its ground state, copper has the electron configuration of [ar]4s 2 3d 9. The full electron configuration for copper is [ar] 3d10 4s1. Since 1s can only hold two electrons the next 2. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. There are two valence electrons in. Copper Electron Configuration Orbital.
From www.alamy.com
Copper (Cu). Diagram of the valence orbitals of an atom of copper64 Copper Electron Configuration Orbital Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The full electron configuration for copper is [ar] 3d10 4s1. In writing the electron configuration for. Copper Electron Configuration Orbital.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electron Configuration Orbital This means that the 29 electrons are distributed among the various orbitals available in the copper atom. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. In writing the electron configuration for copper the first two electrons will go in the. Copper Electron Configuration Orbital.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Orbital In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The full electron configuration for copper is [ar] 3d10 4s1. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the. Copper Electron Configuration Orbital.
From fity.club
Electron Configuration Of Copper Copper Electron Configuration Orbital The full electron configuration for copper is [ar] 3d10 4s1. What is the electron configuration of copper? This means that the 29 electrons are distributed among the various orbitals available in the copper atom. Electron configuration describes the distribution of electrons within an atom’s orbitals. The electron distribution in copper spans across the k, l, m, and n shells, with. Copper Electron Configuration Orbital.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Configuration Orbital There are two valence electrons in the copper. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The full electron configuration for copper is [ar] 3d10 4s1. Electron configuration describes the distribution of electrons within an atom’s orbitals. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Since 1s can. Copper Electron Configuration Orbital.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electron Configuration Orbital In writing the electron configuration for copper the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. This means that the 29 electrons are distributed among the various orbitals available in the. Copper Electron Configuration Orbital.
From fity.club
Electron Configuration Of Copper Copper Electron Configuration Orbital There are two valence electrons in the copper. Copper’s electron configuration is [ar] 3d¹⁰ 4s¹. Electron configuration describes the distribution of electrons within an atom’s orbitals. The full electron configuration for copper is [ar] 3d10 4s1. This means that the 29 electrons are distributed among the various orbitals available in the copper atom. 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Copper Electron Configuration Orbital.