Dilute Solution With Hydrochloric Acid . In strong acidic solution, when the acidic solution is diluted, h 3 o +. Once you understand this relationship, the calculations are simple. C f x v f = c s x v s. C f (concentration of diluted. Preparing dilutions is a common activity in the chemistry lab and elsewhere. We will begin our discussion of solution concentration with two related and relative terms: Ml of a 2.0 m solution of. Imagine you have a concentrated solution of hydrochloric acid. You can use this calculator to determine how much of it you need if you want. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. We can dilute any solution by adding strong acid to the distilled water. To dilute a solution of concentrated acid or. The dilute acid converts the base such as. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. A dilute solution is one.
from docslib.org
We will begin our discussion of solution concentration with two related and relative terms: The dilute acid converts the base such as. Ml of a 2.0 m solution of. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. You can use this calculator to determine how much of it you need if you want. Preparing dilutions is a common activity in the chemistry lab and elsewhere. A dilute solution is one. In strong acidic solution, when the acidic solution is diluted, h 3 o +. We can dilute any solution by adding strong acid to the distilled water.
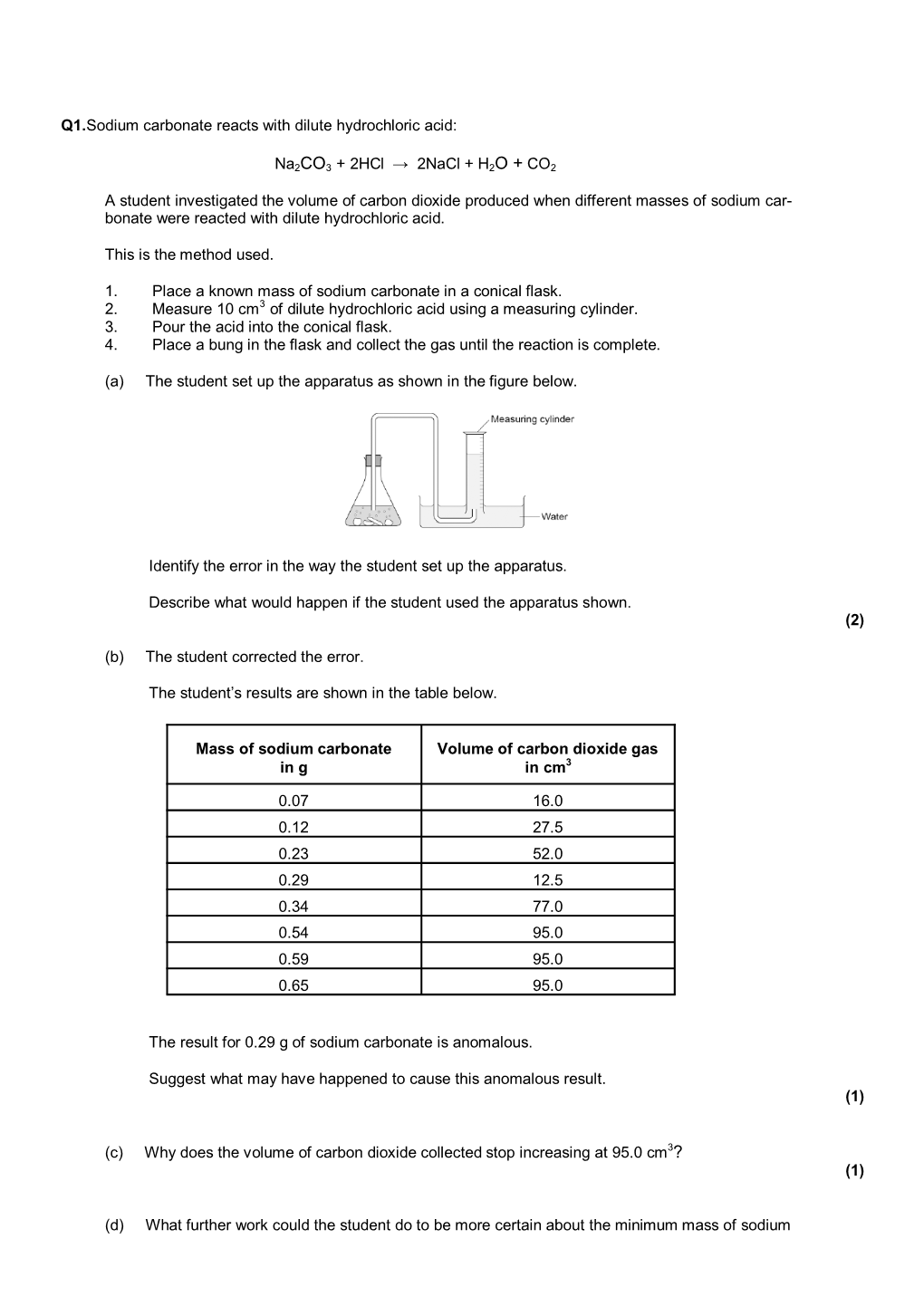
Q1.Sodium Carbonate Reacts with Dilute Hydrochloric Acid DocsLib
Dilute Solution With Hydrochloric Acid We will begin our discussion of solution concentration with two related and relative terms: We can dilute any solution by adding strong acid to the distilled water. To dilute a solution of concentrated acid or. You can use this calculator to determine how much of it you need if you want. Imagine you have a concentrated solution of hydrochloric acid. A dilute solution is one. Once you understand this relationship, the calculations are simple. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. In strong acidic solution, when the acidic solution is diluted, h 3 o +. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. The dilute acid converts the base such as. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Dilution method is described in detail in the post “ preparing working solution from stock solution by dilution ”. C f x v f = c s x v s. We will begin our discussion of solution concentration with two related and relative terms: C f (concentration of diluted.
From www.indiamart.com
Dilute Hydrochloric Acid, For Laboratory, 99 Pure at Rs 300/litre in Dilute Solution With Hydrochloric Acid You can use this calculator to determine how much of it you need if you want. Imagine you have a concentrated solution of hydrochloric acid. C f (concentration of diluted. A dilute solution is one. We can dilute any solution by adding strong acid to the distilled water. The dilute acid converts the base such as. Once you understand this. Dilute Solution With Hydrochloric Acid.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 5 PowerPoint Presentation, free Dilute Solution With Hydrochloric Acid In strong acidic solution, when the acidic solution is diluted, h 3 o +. We can dilute any solution by adding strong acid to the distilled water. You can use this calculator to determine how much of it you need if you want. C f x v f = c s x v s. We will begin our discussion of. Dilute Solution With Hydrochloric Acid.
From askfilo.com
Distilled water (II) Glucose (III) Dilute hydrochloric acid (IV)Sodium h.. Dilute Solution With Hydrochloric Acid You can use this calculator to determine how much of it you need if you want. Imagine you have a concentrated solution of hydrochloric acid. In strong acidic solution, when the acidic solution is diluted, h 3 o +. Ml of a 2.0 m solution of. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures. Dilute Solution With Hydrochloric Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Dilute Solution With Hydrochloric Acid Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. Preparing dilutions is a common activity in the chemistry lab and elsewhere. The dilute acid converts the base such as. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. Imagine you have. Dilute Solution With Hydrochloric Acid.
From www.gkseries.com
Which of the following are present in a dilute Aqueous solution of Dilute Solution With Hydrochloric Acid We will begin our discussion of solution concentration with two related and relative terms: To dilute a solution of concentrated acid or. C f (concentration of diluted. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. C f x v f = c s x v s. Ml of a 2.0 m solution. Dilute Solution With Hydrochloric Acid.
From www.lazada.com.my
Bolinda dilute hydrochloric acid standard solution laboratory HCL water Dilute Solution With Hydrochloric Acid To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. We can dilute any solution by adding strong acid to the distilled water. Dilution method is described in detail in the post “ preparing working solution from stock solution by dilution ”. You can use this calculator to determine how much of it you. Dilute Solution With Hydrochloric Acid.
From www.sciencephoto.com
Dilute hydrochloric acid reactions Stock Image A500/0552 Science Dilute Solution With Hydrochloric Acid In strong acidic solution, when the acidic solution is diluted, h 3 o +. You can use this calculator to determine how much of it you need if you want. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. To dilute a solution of concentrated acid or. C. Dilute Solution With Hydrochloric Acid.
From www.alamy.com
Reactivity of metals. Magnesium, zinc, iron and lead in dilute Dilute Solution With Hydrochloric Acid To dilute a solution of concentrated acid or. In strong acidic solution, when the acidic solution is diluted, h 3 o +. You can use this calculator to determine how much of it you need if you want. A dilute solution is one. Imagine you have a concentrated solution of hydrochloric acid. Once you understand this relationship, the calculations are. Dilute Solution With Hydrochloric Acid.
From www.sciencephoto.com
Bottle of dilute hydrochloric acid Stock Image C019/8335 Science Dilute Solution With Hydrochloric Acid To dilute a solution of concentrated acid or. A dilute solution is one. Preparing dilutions is a common activity in the chemistry lab and elsewhere. The dilute acid converts the base such as. Ml of a 2.0 m solution of. We can dilute any solution by adding strong acid to the distilled water. Use the following formula to calculate the. Dilute Solution With Hydrochloric Acid.
From www.alamy.com
Standard dilute solution of hydrochloric acid is 1.0M. Under the Dilute Solution With Hydrochloric Acid The dilute acid converts the base such as. C f (concentration of diluted. You can use this calculator to determine how much of it you need if you want. Imagine you have a concentrated solution of hydrochloric acid. In strong acidic solution, when the acidic solution is diluted, h 3 o +. We can dilute any solution by adding strong. Dilute Solution With Hydrochloric Acid.
From www.numerade.com
In an experiment, equal volumes of dilute hydrochloric acid (solution A Dilute Solution With Hydrochloric Acid The dilute acid converts the base such as. Preparing dilutions is a common activity in the chemistry lab and elsewhere. We will begin our discussion of solution concentration with two related and relative terms: We can dilute any solution by adding strong acid to the distilled water. C f (concentration of diluted. To dilute a solution of concentrated acid or.. Dilute Solution With Hydrochloric Acid.
From sciencing.com
How to Make a 50 Normal Solution of Hydrochloric Acid Sciencing Dilute Solution With Hydrochloric Acid We will begin our discussion of solution concentration with two related and relative terms: In strong acidic solution, when the acidic solution is diluted, h 3 o +. The dilute acid converts the base such as. Once you understand this relationship, the calculations are simple. To dilute a solution of concentrated acid or. Imagine you have a concentrated solution of. Dilute Solution With Hydrochloric Acid.
From www.youtube.com
How to prepare dilute solution of hydrochloric acid (HCl) in laboratory Dilute Solution With Hydrochloric Acid Once you understand this relationship, the calculations are simple. A dilute solution is one. Imagine you have a concentrated solution of hydrochloric acid. We can dilute any solution by adding strong acid to the distilled water. Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1m solution of hydrochloric acid. Dilute Solution With Hydrochloric Acid.
From brainly.in
Explain the action of dilute hydrochloric acid on the following with Dilute Solution With Hydrochloric Acid To dilute a solution of concentrated acid or. The dilute acid converts the base such as. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. You can use this calculator to determine how much of it you need if you want. We will begin our discussion of solution. Dilute Solution With Hydrochloric Acid.
From www.lazada.com.ph
Dilute hydrochloric acid standard solution 0.1 0.2 0.5 1.0mol molar Dilute Solution With Hydrochloric Acid We will begin our discussion of solution concentration with two related and relative terms: We can dilute any solution by adding strong acid to the distilled water. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Imagine you have a concentrated solution of hydrochloric acid. Once you understand this relationship, the calculations are simple. Ml of a. Dilute Solution With Hydrochloric Acid.
From www.youtube.com
Q.11 What happens when Dilute Hydrochloric acid is added to iron Dilute Solution With Hydrochloric Acid C f (concentration of diluted. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. The dilute acid converts the base such as. Imagine you have a concentrated solution of hydrochloric acid. You can use this calculator to determine how much of it you need if you want. Once you understand this relationship, the. Dilute Solution With Hydrochloric Acid.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Dilute Solution With Hydrochloric Acid To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. Ml of a 2.0 m solution of. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Imagine you have a concentrated solution of hydrochloric acid. Dilute hydrochloric acid is often used in the extraction. Dilute Solution With Hydrochloric Acid.
From niasrfrank.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid NiasrFrank Dilute Solution With Hydrochloric Acid Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1m solution of hydrochloric acid solution. Ml of a 2.0 m solution of. We can dilute any solution by adding. Dilute Solution With Hydrochloric Acid.
From www.nagwa.com
Question Video Determining the Precipitate Color and the Identity of Dilute Solution With Hydrochloric Acid C f (concentration of diluted. To dilute a solution of concentrated acid or. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. Imagine you have a concentrated solution of hydrochloric acid. Dilution method is described in detail in the post “ preparing working solution from stock solution by dilution ”. Ml of a. Dilute Solution With Hydrochloric Acid.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn Dilute Solution With Hydrochloric Acid Dilution method is described in detail in the post “ preparing working solution from stock solution by dilution ”. We will begin our discussion of solution concentration with two related and relative terms: To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. In strong acidic solution, when the acidic solution is diluted, h. Dilute Solution With Hydrochloric Acid.
From childhealthpolicy.vumc.org
🌷 How to make dilute hcl. Allotropes of sulfur. 20221011 Dilute Solution With Hydrochloric Acid C f x v f = c s x v s. C f (concentration of diluted. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. Preparing dilutions is a common activity in the chemistry lab and elsewhere. We will begin our discussion of solution concentration with two related and relative terms: You can. Dilute Solution With Hydrochloric Acid.
From www.sciencecompany.com
Hydrochloric Acid, 10 solution, 250ml for sale. Buy from The Science Dilute Solution With Hydrochloric Acid Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. Imagine you have a concentrated solution of hydrochloric acid. The dilute acid converts the base such as. C f x v f = c s x v s. To dilute a solution of concentrated acid or. C f (concentration. Dilute Solution With Hydrochloric Acid.
From docslib.org
Q1.Sodium Carbonate Reacts with Dilute Hydrochloric Acid DocsLib Dilute Solution With Hydrochloric Acid Ml of a 2.0 m solution of. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. We will begin our discussion of solution concentration with two related and relative terms: Once you understand this relationship, the calculations are simple. To prepare a solution of specific molarity based on. Dilute Solution With Hydrochloric Acid.
From www.alamy.com
calcium carbonate reacts with dilute hydrochloric acid to produce Stock Dilute Solution With Hydrochloric Acid Dilution method is described in detail in the post “ preparing working solution from stock solution by dilution ”. The dilute acid converts the base such as. Ml of a 2.0 m solution of. We can dilute any solution by adding strong acid to the distilled water. C f x v f = c s x v s. A dilute. Dilute Solution With Hydrochloric Acid.
From www.chegg.com
Solved 1 Dilute hydrochloric acid reacts with sodium Dilute Solution With Hydrochloric Acid A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: Ml of a 2.0 m solution of. Once you understand this relationship, the calculations are simple. Imagine you have a concentrated solution of hydrochloric acid. We can dilute any solution by adding strong acid to the distilled water. Use the following. Dilute Solution With Hydrochloric Acid.
From www.calpaclab.com
Hydrochloric Acid, Dilute R, 73 g/L HCl, 1 Liter Dilute Solution With Hydrochloric Acid Imagine you have a concentrated solution of hydrochloric acid. A dilute solution is one. Once you understand this relationship, the calculations are simple. To dilute a solution of concentrated acid or. Ml of a 2.0 m solution of. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. In. Dilute Solution With Hydrochloric Acid.
From www.slideserve.com
PPT The reaction of magnesium oxide with dilute hydrochloric acid Dilute Solution With Hydrochloric Acid In strong acidic solution, when the acidic solution is diluted, h 3 o +. Imagine you have a concentrated solution of hydrochloric acid. A dilute solution is one. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. The dilute acid converts the base such as. We can dilute any solution by adding strong. Dilute Solution With Hydrochloric Acid.
From melscience.com
Hydrochloric acid, solution 2 M MEL Chemistry Dilute Solution With Hydrochloric Acid Preparing dilutions is a common activity in the chemistry lab and elsewhere. To dilute a solution of concentrated acid or. We will begin our discussion of solution concentration with two related and relative terms: C f x v f = c s x v s. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or. Dilute Solution With Hydrochloric Acid.
From weston-kgrimes.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid Dilute Solution With Hydrochloric Acid The dilute acid converts the base such as. To dilute a solution of concentrated acid or. Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1m solution of hydrochloric acid solution. Imagine you have a concentrated solution of hydrochloric acid. C f (concentration of diluted. In strong acidic solution, when. Dilute Solution With Hydrochloric Acid.
From www.sciencephoto.com
Bottle of dilute hydrochloric acid Stock Image C019/8337 Science Dilute Solution With Hydrochloric Acid C f x v f = c s x v s. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. We can dilute any solution by adding strong acid to the distilled water. You can use. Dilute Solution With Hydrochloric Acid.
From medicolab.africa
Dilute Hydrochloric Acid (100ml) MedicoLab Dilute Solution With Hydrochloric Acid We can dilute any solution by adding strong acid to the distilled water. The dilute acid converts the base such as. A dilute solution is one. Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1m solution of hydrochloric acid solution. C f x v f = c s x. Dilute Solution With Hydrochloric Acid.
From www.lazada.com.ph
Dilute hydrochloric acid standard solution 0.1 0.2 0.5 1.0mol molar Dilute Solution With Hydrochloric Acid C f x v f = c s x v s. Use the following formula to calculate the volume of concentrated solution required for the preparation of 1 liter of 1m solution of hydrochloric acid solution. Imagine you have a concentrated solution of hydrochloric acid. Dilution method is described in detail in the post “ preparing working solution from stock. Dilute Solution With Hydrochloric Acid.
From www.doubtnut.com
Chemical Tests between Dilute hydrochloric acid and dilute sulphur Dilute Solution With Hydrochloric Acid To dilute a solution of concentrated acid or. Imagine you have a concentrated solution of hydrochloric acid. Once you understand this relationship, the calculations are simple. C f x v f = c s x v s. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. We can. Dilute Solution With Hydrochloric Acid.
From www.youtube.com
What Happens when Magnesium reacts with Acetic Acid And Dilute Dilute Solution With Hydrochloric Acid Preparing dilutions is a common activity in the chemistry lab and elsewhere. To prepare a solution of specific molarity based on mass, please use the mass molarity calculator. Dilute hydrochloric acid is often used in the extraction of basic substances from mixtures or in the removal of basic impurities. Dilution method is described in detail in the post “ preparing. Dilute Solution With Hydrochloric Acid.
From www.youtube.com
How to prepared dilute solution of HCl/Hydrochloric acid YouTube Dilute Solution With Hydrochloric Acid The dilute acid converts the base such as. Once you understand this relationship, the calculations are simple. Preparing dilutions is a common activity in the chemistry lab and elsewhere. C f (concentration of diluted. Imagine you have a concentrated solution of hydrochloric acid. Dilution method is described in detail in the post “ preparing working solution from stock solution by. Dilute Solution With Hydrochloric Acid.