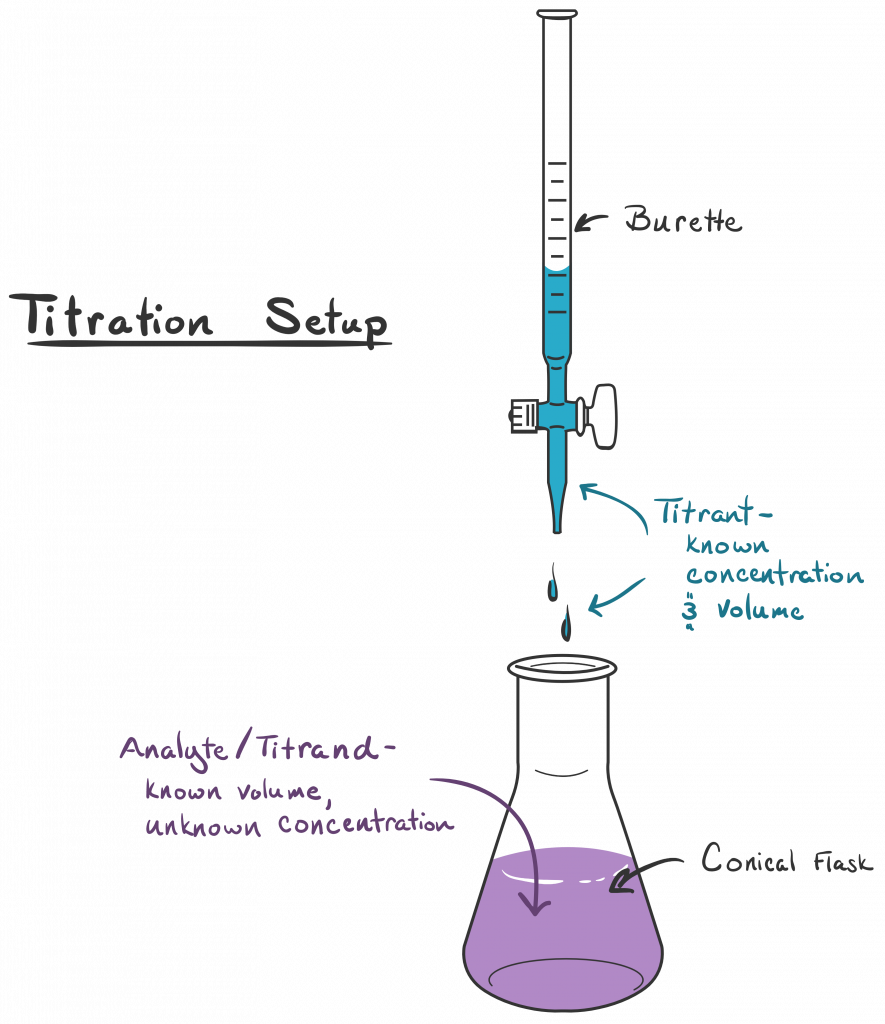
What Is Titration In Chemistry Easy Definition . For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a.
from www.microlit.com
Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration. The basic process involves adding a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.
An Advanced Guide to Titration Microlit
What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. The basic process involves adding a. For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.
From mmerevise.co.uk
Titrations and Uncertainties MME What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant). What Is Titration In Chemistry Easy Definition.
From chemistnotes.com
Conductometric titration, easy principle, curves, 3 advantages What Is Titration In Chemistry Easy Definition The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant,. What Is Titration In Chemistry Easy Definition.
From cecbzmbb.blob.core.windows.net
Definition And Example Of Titration at Arlene Hall blog What Is Titration In Chemistry Easy Definition The basic process involves adding a. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. For example, you can use it to find the concentration. Titration is. What Is Titration In Chemistry Easy Definition.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution. What Is Titration In Chemistry Easy Definition.
From www.youtube.com
TYPES OF TITRATION IN CHEMISTRY ACID BASE TITRATION REDOX TITRATION What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration. Titration involves the gradual addition of a. What Is Titration In Chemistry Easy Definition.
From dxobyswdg.blob.core.windows.net
Titration Means What In Chemistry at Sabrina Reyes blog What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the process in which one solution is added to another solution such that it. What Is Titration In Chemistry Easy Definition.
From www.scribd.com
Titration PDF Titration Chemistry What Is Titration In Chemistry Easy Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. For example, you can use it to find the concentration. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added.. What Is Titration In Chemistry Easy Definition.
From www.science-revision.co.uk
Titrations What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is. What Is Titration In Chemistry Easy Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is Titration In Chemistry Easy Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the process in which one solution is added to another solution. What Is Titration In Chemistry Easy Definition.
From www.youtube.com
Titration GCSE Chemistry YouTube What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the. What Is Titration In Chemistry Easy Definition.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined,. What Is Titration In Chemistry Easy Definition.
From www.pinterest.com
Titration Easy Science Learn biology, Cool science facts, Ap chemistry What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a. What Is Titration In Chemistry Easy Definition.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a. What Is Titration In Chemistry Easy Definition.
From theedge.com.hk
Chemistry How To Titration The Edge What Is Titration In Chemistry Easy Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration. What Is Titration In Chemistry Easy Definition.
From letitsnowglobe.co.uk
Titration procedure pdf What Is Titration In Chemistry Easy Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration. What Is Titration In Chemistry Easy Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration. What Is Titration In Chemistry Easy Definition.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and What Is Titration In Chemistry Easy Definition The basic process involves adding a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the. What Is Titration In Chemistry Easy Definition.
From fity.club
Titration What Is Titration In Chemistry Easy Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The basic process involves adding a. A titration is a. What Is Titration In Chemistry Easy Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the slow addition of one solution of a known concentration. What Is Titration In Chemistry Easy Definition.
From mavink.com
Titration Diagram Labeled What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow. What Is Titration In Chemistry Easy Definition.
From dxobyswdg.blob.core.windows.net
Titration Means What In Chemistry at Sabrina Reyes blog What Is Titration In Chemistry Easy Definition The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a. What Is Titration In Chemistry Easy Definition.
From mavink.com
Types Of Acid Base Titration What Is Titration In Chemistry Easy Definition The basic process involves adding a. For example, you can use it to find the concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the process in which one solution is added to another solution such that it reacts. What Is Titration In Chemistry Easy Definition.
From dxolbvlxr.blob.core.windows.net
Titration Experiment Method at Cheryl Hendrix blog What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. What Is Titration In Chemistry Easy Definition.
From sachiacidbase.weebly.com
Titrations Sachi's Acids and Bases What Is Titration In Chemistry Easy Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. A titration is a laboratory technique used. What Is Titration In Chemistry Easy Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is Titration In Chemistry Easy Definition The basic process involves adding a. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the. What Is Titration In Chemistry Easy Definition.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators What Is Titration In Chemistry Easy Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The basic process involves adding a. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration is the process in which one solution. What Is Titration In Chemistry Easy Definition.
From www.youtube.com
Titration Acid Base Titration Chemistry YouTube What Is Titration In Chemistry Easy Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration. A titration is a laboratory technique used. What Is Titration In Chemistry Easy Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog What Is Titration In Chemistry Easy Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. The basic process involves adding a. Titration is the slow. What Is Titration In Chemistry Easy Definition.
From dxotuvjtw.blob.core.windows.net
What Is An Indicator In Chemistry Titration at Edward Mcraney blog What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. For example, you can use it to find the concentration. Titration is a way of. What Is Titration In Chemistry Easy Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The basic process involves. What Is Titration In Chemistry Easy Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is Titration In Chemistry Easy Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known. The basic process involves adding a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to. What Is Titration In Chemistry Easy Definition.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Is Titration In Chemistry Easy Definition The basic process involves adding a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added. A titration is a laboratory technique used to precisely measure molar concentration. What Is Titration In Chemistry Easy Definition.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog What Is Titration In Chemistry Easy Definition For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a.. What Is Titration In Chemistry Easy Definition.
From corinneafestuart.blogspot.com
Titration Apparatus What Is Titration In Chemistry Easy Definition The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine. What Is Titration In Chemistry Easy Definition.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 What Is Titration In Chemistry Easy Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the process in which. What Is Titration In Chemistry Easy Definition.