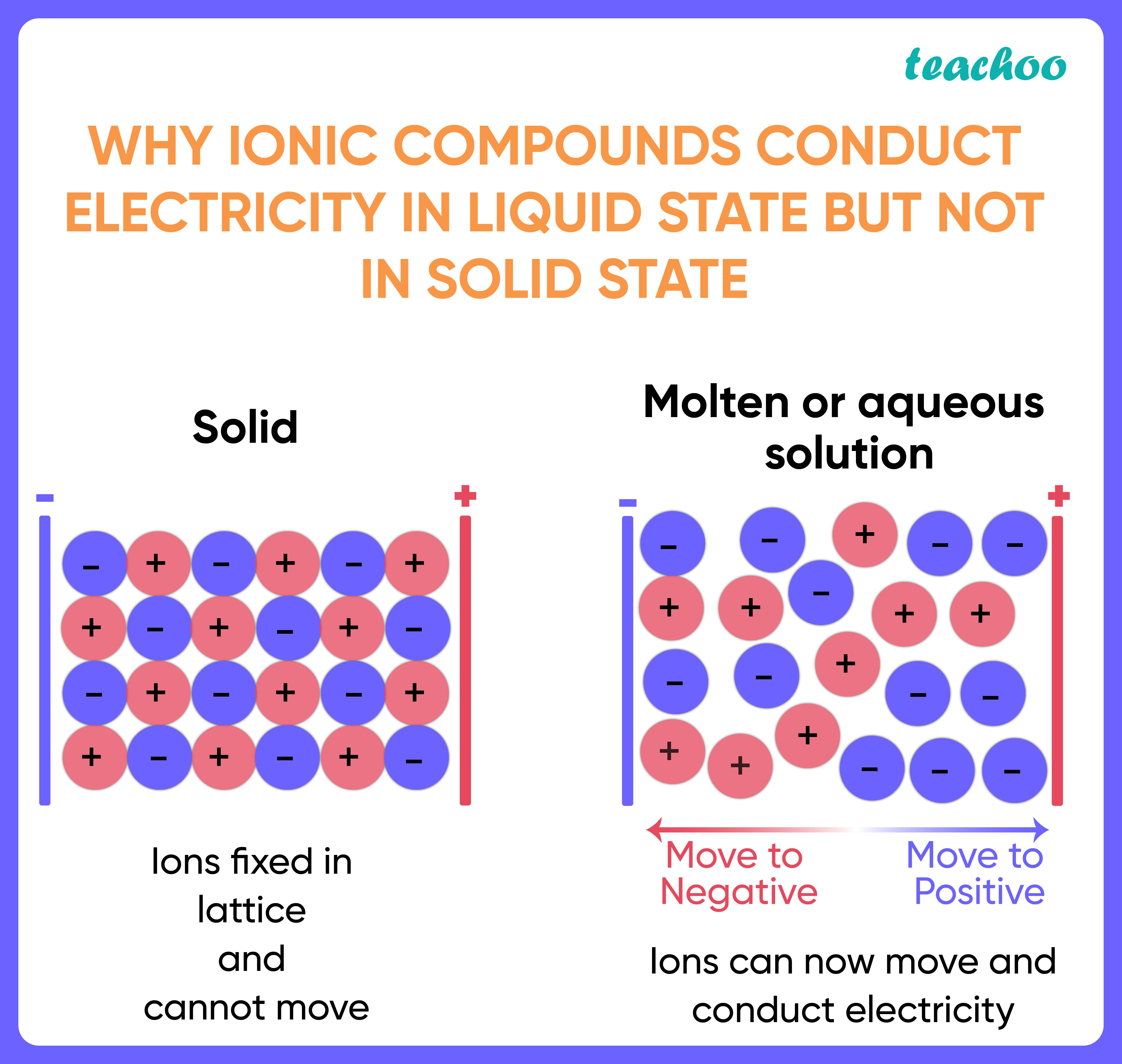
Electrical Conductivity Of Simple Molecular Substances . As the molecules are not physically connected there should be no electrical conductivity under any conditions. Simple molecular structures are poor conductors of electricity (even when molten) because: There are no free ions or electrons to move and carry. They are also soft, again due to the weak intermolecular forces. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Molecular substances won't conduct electricity. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Simple molecular substances have low melting and boiling points, and do not conduct electricity. Substances can be explained by thinking about. The low melting point of y suggests that little.
from www.teachoo.com
There are no free ions or electrons to move and carry. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Substances can be explained by thinking about. Simple molecular substances have low melting and boiling points, and do not conduct electricity. Molecular substances won't conduct electricity. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. The low melting point of y suggests that little. They are also soft, again due to the weak intermolecular forces. Simple molecular structures are poor conductors of electricity (even when molten) because:
The table shown below gives information about four Chemistry MCQ
Electrical Conductivity Of Simple Molecular Substances Simple molecular structures are poor conductors of electricity (even when molten) because: Molecular substances won't conduct electricity. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Simple molecular substances have low melting and boiling points, and do not conduct electricity. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. As the molecules are not physically connected there should be no electrical conductivity under any conditions. There are no free ions or electrons to move and carry. The low melting point of y suggests that little. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. They are also soft, again due to the weak intermolecular forces. Substances can be explained by thinking about. Simple molecular structures are poor conductors of electricity (even when molten) because: The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure.
From www.slideserve.com
PPT Chapter 9 Chemical Change PowerPoint Presentation, free download Electrical Conductivity Of Simple Molecular Substances Substances can be explained by thinking about. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Molecular substances won't conduct electricity. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. They are also soft, again due to the weak intermolecular forces. Simple molecular structures. Electrical Conductivity Of Simple Molecular Substances.
From www.hanlin.com
IB DP Chemistry SL复习笔记4.3.3 Properties of Covalent Compounds翰林国际教育 Electrical Conductivity Of Simple Molecular Substances There are no free ions or electrons to move and carry. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. Simple molecular substances have low melting and boiling points, and do not conduct electricity. Molecular substances won't conduct electricity. The low melting point of y suggests that little.. Electrical Conductivity Of Simple Molecular Substances.
From www.researchgate.net
Thermal conductivity versus electrical conductivity times temperature Electrical Conductivity Of Simple Molecular Substances There are no free ions or electrons to move and carry. The low melting point of y suggests that little. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Substances can be explained by thinking about. Simple molecular structures are poor conductors of electricity (even when molten) because: They are also soft, again. Electrical Conductivity Of Simple Molecular Substances.
From www.slideserve.com
PPT Chemical and Physical Properties PowerPoint Presentation, free Electrical Conductivity Of Simple Molecular Substances They are also soft, again due to the weak intermolecular forces. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. Molecular substances won't conduct electricity. Simple molecular structures are poor conductors of. Electrical Conductivity Of Simple Molecular Substances.
From www.slideserve.com
PPT Solutions Chapter 12 Modern Chemistry PowerPoint Presentation Electrical Conductivity Of Simple Molecular Substances The low melting point of y suggests that little. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Simple molecular structures are poor conductors of electricity (even when molten) because: Simple molecular substances have low melting and boiling points, and do not conduct electricity. Molecular substances won't conduct electricity. Molecular compounds are poor. Electrical Conductivity Of Simple Molecular Substances.
From slideplayer.com
Properties of Substances ppt download Electrical Conductivity Of Simple Molecular Substances The low melting point of y suggests that little. Simple molecular structures are poor conductors of electricity (even when molten) because: They are also soft, again due to the weak intermolecular forces. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Simple molecular substances have low melting and boiling points, and do not. Electrical Conductivity Of Simple Molecular Substances.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Electrical Conductivity Of Simple Molecular Substances Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Simple molecular structures are poor conductors of electricity (even when molten) because: The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. The low melting point of y suggests that little.. Electrical Conductivity Of Simple Molecular Substances.
From www.thoughtco.com
Electrical Conductivity of Metals Electrical Conductivity Of Simple Molecular Substances At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Substances can be explained by thinking about. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. The low melting point of y suggests that little. Molecular compounds are poor conductors of electricity as there are. Electrical Conductivity Of Simple Molecular Substances.
From www.slideshare.net
Chem matters ch7_covalent_bonding Electrical Conductivity Of Simple Molecular Substances The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Substances can be explained by thinking about. Molecular substances won't conduct electricity. Molecular compounds are poor conductors of electricity as there are no. Electrical Conductivity Of Simple Molecular Substances.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Periodicity Electrical Conducitiy Trend Electrical Conductivity Of Simple Molecular Substances The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. Simple molecular substances have low melting and boiling points, and do not conduct electricity. Simple molecular structures are poor conductors of electricity (even when molten) because: Substances can be explained by thinking about. There are no free ions or. Electrical Conductivity Of Simple Molecular Substances.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University Electrical Conductivity Of Simple Molecular Substances At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Molecular substances won't conduct electricity. Simple molecular substances have low melting and boiling points, and do not conduct electricity. There are no free ions or electrons to move and carry. The relatively high melting point, solubility in water and electrical conductivity when. Electrical Conductivity Of Simple Molecular Substances.
From mungfali.com
Electrical Conductivity Explained Electrical Conductivity Of Simple Molecular Substances Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. The low melting point of y suggests that little. There are no free ions or electrons to move and carry. Substances can be explained by thinking. Electrical Conductivity Of Simple Molecular Substances.
From www.linstitute.net
IB DP Chemistry HL复习笔记4.2.5 Giant Covalent Structures翰林国际教育 Electrical Conductivity Of Simple Molecular Substances They are also soft, again due to the weak intermolecular forces. Simple molecular structures are poor conductors of electricity (even when molten) because: Substances can be explained by thinking about. There are no free ions or electrons to move and carry. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. The. Electrical Conductivity Of Simple Molecular Substances.
From ec.bertabulle.com
How Do Ions Conduct Electricity Electrical Conductivity Of Simple Molecular Substances Substances can be explained by thinking about. Molecular substances won't conduct electricity. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. As the molecules are not physically connected there should be no electrical conductivity under any conditions. There are no free ions or electrons to move and carry. Simple molecular structures. Electrical Conductivity Of Simple Molecular Substances.
From www.nagwa.com
Question Video Identifying When Ionic Substances Are Conductive Nagwa Electrical Conductivity Of Simple Molecular Substances Molecular substances won't conduct electricity. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. There are no free ions or electrons to move and carry. Substances can be explained by thinking about. As the molecules are not physically connected there should be no electrical conductivity under any conditions.. Electrical Conductivity Of Simple Molecular Substances.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog Electrical Conductivity Of Simple Molecular Substances Simple molecular substances have low melting and boiling points, and do not conduct electricity. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. The low melting point of y suggests that little. Molecular substances won't. Electrical Conductivity Of Simple Molecular Substances.
From www.researchgate.net
Electrical conductivity (volume) of metalreinforced plastic composites Electrical Conductivity Of Simple Molecular Substances Simple molecular substances have low melting and boiling points, and do not conduct electricity. They are also soft, again due to the weak intermolecular forces. There are no free ions or electrons to move and carry. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. As the molecules are not physically connected there. Electrical Conductivity Of Simple Molecular Substances.
From gamma.app
Why Simple Molecular Compounds Have Low Melting and Boiling Points, and Electrical Conductivity Of Simple Molecular Substances They are also soft, again due to the weak intermolecular forces. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. As the molecules are not physically connected there should be no electrical conductivity under any conditions. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that. Electrical Conductivity Of Simple Molecular Substances.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Electrical Conductivity Of Simple Molecular Substances Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Simple molecular structures are poor conductors of electricity (even when molten) because: Substances can be explained by thinking about. Molecular substances won't conduct electricity. As the molecules are not physically connected there should be no electrical conductivity under any conditions. There are no free. Electrical Conductivity Of Simple Molecular Substances.
From animalia-life.club
Covalent Network Solids Electrical Conductivity Of Simple Molecular Substances They are also soft, again due to the weak intermolecular forces. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. The low melting point of y suggests that little. Molecular compounds are poor conductors of. Electrical Conductivity Of Simple Molecular Substances.
From slideplayer.com
Simple molecular substances ppt download Electrical Conductivity Of Simple Molecular Substances As the molecules are not physically connected there should be no electrical conductivity under any conditions. Simple molecular substances have low melting and boiling points, and do not conduct electricity. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. There are no free ions or electrons to move. Electrical Conductivity Of Simple Molecular Substances.
From chem.libretexts.org
2.1.1 Comparing Ionic and Molecular Substances Chemistry LibreTexts Electrical Conductivity Of Simple Molecular Substances Simple molecular structures are poor conductors of electricity (even when molten) because: At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Molecular substances won't conduct electricity. Simple molecular substances have low melting and boiling points, and do not conduct electricity. The relatively high melting point, solubility in water and electrical conductivity. Electrical Conductivity Of Simple Molecular Substances.
From www.researchgate.net
Electrical conductivity of different materials at 300 K. Download Electrical Conductivity Of Simple Molecular Substances Simple molecular structures are poor conductors of electricity (even when molten) because: Substances can be explained by thinking about. The low melting point of y suggests that little. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. The relatively high melting point, solubility in water and electrical conductivity when molten suggest. Electrical Conductivity Of Simple Molecular Substances.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Electrical Conductivity Of Simple Molecular Substances Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Substances can be explained by thinking about. As the molecules are not physically connected there should be no electrical conductivity under any conditions. The low melting point of y suggests that little. Simple molecular structures are poor conductors of electricity (even when. Electrical Conductivity Of Simple Molecular Substances.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog Electrical Conductivity Of Simple Molecular Substances Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Simple molecular substances have low melting and boiling points, and do not conduct electricity. There are no free ions or electrons to move and carry. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. The. Electrical Conductivity Of Simple Molecular Substances.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Electrical Conductivity Of Simple Molecular Substances Molecular substances won't conduct electricity. They are also soft, again due to the weak intermolecular forces. There are no free ions or electrons to move and carry. Simple molecular substances have low melting and boiling points, and do not conduct electricity. The low melting point of y suggests that little. As the molecules are not physically connected there should be. Electrical Conductivity Of Simple Molecular Substances.
From www.electricity-magnetism.org
Conductivity Sensors How it works, Application & Advantages Electrical Conductivity Of Simple Molecular Substances The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. As the molecules are not physically connected there should be no electrical conductivity under any conditions. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Simple molecular structures are poor. Electrical Conductivity Of Simple Molecular Substances.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Electrical Conductivity Of Simple Molecular Substances Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. There are no free ions or electrons to move and carry. Substances can be explained by thinking about. The low melting point of y suggests that little. As the molecules are not physically connected there should be no electrical conductivity under any conditions. At. Electrical Conductivity Of Simple Molecular Substances.
From socratic.org
How can you use electrical conductivity to decide if a compound is Electrical Conductivity Of Simple Molecular Substances Molecular substances won't conduct electricity. They are also soft, again due to the weak intermolecular forces. Substances can be explained by thinking about. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Simple molecular substances have low melting and boiling points, and do not conduct electricity. As the molecules are not. Electrical Conductivity Of Simple Molecular Substances.
From taylorsciencegeeks.weebly.com
Methods of Heat Transfer Conduction Science News Electrical Conductivity Of Simple Molecular Substances The low melting point of y suggests that little. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Substances can be explained by thinking about. Molecular substances won't conduct electricity. As the molecules. Electrical Conductivity Of Simple Molecular Substances.
From www.mdpi.com
Molecules Free FullText Dielectric Loss and Electrical Electrical Conductivity Of Simple Molecular Substances At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling points. They are also soft, again due to the weak intermolecular forces. As the molecules are not physically connected there should be no electrical conductivity under any conditions. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient. Electrical Conductivity Of Simple Molecular Substances.
From favpng.com
Ionic Compound Sodium Chloride Electrical Conductivity Water, PNG Electrical Conductivity Of Simple Molecular Substances They are also soft, again due to the weak intermolecular forces. Simple molecular substances have low melting and boiling points, and do not conduct electricity. Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and boiling. Electrical Conductivity Of Simple Molecular Substances.
From www.researchgate.net
Conductivity ranges for different applications [78,79]. Download Electrical Conductivity Of Simple Molecular Substances Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the. Molecular substances won't conduct electricity. The low melting point of y suggests that little. The relatively high melting point, solubility in water and electrical conductivity when molten suggest that x is a giant ionic structure. At room temperature, simple molecular substances are. Electrical Conductivity Of Simple Molecular Substances.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Electrical Conductivity Of Simple Molecular Substances As the molecules are not physically connected there should be no electrical conductivity under any conditions. Even in cases where electrons may be delocalised within a particular molecule, there isn't sufficient contact. Molecular substances won't conduct electricity. Substances can be explained by thinking about. At room temperature, simple molecular substances are gases, or liquids or solids with low melting and. Electrical Conductivity Of Simple Molecular Substances.
From slideplayer.com
Simple molecular substances ppt download Electrical Conductivity Of Simple Molecular Substances Simple molecular substances have low melting and boiling points, and do not conduct electricity. There are no free ions or electrons to move and carry. They are also soft, again due to the weak intermolecular forces. Molecular substances won't conduct electricity. Substances can be explained by thinking about. Molecular compounds are poor conductors of electricity as there are no free. Electrical Conductivity Of Simple Molecular Substances.