Calculate Heat Evolved . It is no more difficult than that! Calculate the molar enthalpy of formation from combustion data using hess's law; The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. To understand how enthalpy pertains to chemical reactions. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Define enthalpy and explain its classification as a state function. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. We have stated that the change in energy (\ (δu\)). Since h is defined by the equation:. State the first law of thermodynamics. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction.
from www.youtube.com
To understand how enthalpy pertains to chemical reactions. It is no more difficult than that! We have stated that the change in energy (\ (δu\)). Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Since h is defined by the equation:. Define enthalpy and explain its classification as a state function. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. Calculate the molar enthalpy of formation from combustion data using hess's law; State the first law of thermodynamics.
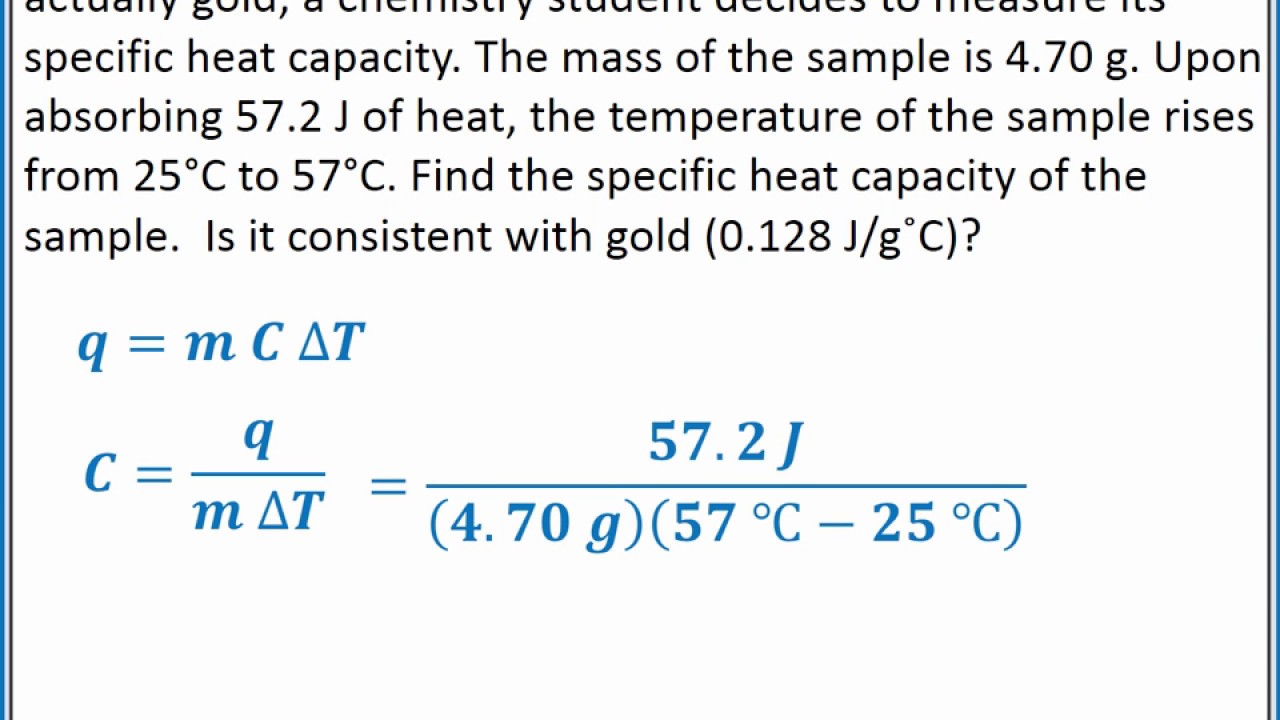
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube
Calculate Heat Evolved This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. State the first law of thermodynamics. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Define enthalpy and explain its classification as a state function. To understand how enthalpy pertains to chemical reactions. It is no more difficult than that! Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. Calculate the molar enthalpy of formation from combustion data using hess's law; We have stated that the change in energy (\ (δu\)). Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Since h is defined by the equation:.
From www.youtube.com
Calculate amount of heat evolved when500 cm^(3) of 0.5 hydrocloric acid is mixed with 200 cm^(3 Calculate Heat Evolved It is no more difficult than that! Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. To understand how enthalpy pertains to chemical reactions. Since h is defined by the equation:. Calculate the molar enthalpy of formation from combustion data. Calculate Heat Evolved.
From www.coursehero.com
[Solved] For trials 1 and 2 calculate the moles, heat evolved, heat capacity... Course Hero Calculate Heat Evolved Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. State the first law of thermodynamics. Define enthalpy and explain its classification as a state function. Heat changes in chemical reactions are often measured in the laboratory under conditions in which. Calculate Heat Evolved.
From www.coursehero.com
[Solved] Calculate the heat evolved (in kJ) upon complete combustion of 3.0... Course Hero Calculate Heat Evolved We have stated that the change in energy (\ (δu\)). This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. It is no more difficult than that! Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Since h is. Calculate Heat Evolved.
From www.slideserve.com
PPT Energy & Chemical Change PowerPoint Presentation, free download ID3528560 Calculate Heat Evolved The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. State the first law of thermodynamics. Calculate the molar enthalpy of formation from combustion data using hess's law; This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion.. Calculate Heat Evolved.
From kassemsonas.blogspot.com
30+ how to calculate heat evolved KassemSonas Calculate Heat Evolved State the first law of thermodynamics. To understand how enthalpy pertains to chemical reactions. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Define enthalpy and explain its classification as a state function. Calculate the molar enthalpy of formation from combustion data using hess's law; Heat changes in chemical reactions. Calculate Heat Evolved.
From www.chegg.com
Solved 26. Calculate the heat evolved when 250 g of oxygen Calculate Heat Evolved The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. We. Calculate Heat Evolved.
From www.numerade.com
SOLVED Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 Calculate Heat Evolved Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. Define enthalpy and explain its classification as a state function. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. To understand how enthalpy pertains to chemical reactions.. Calculate Heat Evolved.
From www.slideserve.com
PPT Reaction Energy and Equilibrium PowerPoint Presentation ID3721064 Calculate Heat Evolved We have stated that the change in energy (\ (δu\)). Since h is defined by the equation:. It is no more difficult than that! To understand how enthalpy pertains to chemical reactions. Define enthalpy and explain its classification as a state function. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in. Calculate Heat Evolved.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calculate Heat Evolved Define enthalpy and explain its classification as a state function. It is no more difficult than that! This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. State the first law of thermodynamics. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Since h. Calculate Heat Evolved.
From www.doubtnut.com
Calculate amount of heat evolved when 500 cm^(3) of 0.5 hydrocloric Calculate Heat Evolved State the first law of thermodynamics. Calculate the molar enthalpy of formation from combustion data using hess's law; Since h is defined by the equation:. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. We have stated that. Calculate Heat Evolved.
From www.slideserve.com
PPT Unit 3 Phase Changes & Energy PowerPoint Presentation ID3721071 Calculate Heat Evolved This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. It is no more difficult than that! Calculate the molar enthalpy of formation from combustion data using hess's law; State the first law of thermodynamics. Enthalpy change is simply a measure of the amount of heat evolved. Calculate Heat Evolved.
From studylib.net
Heat Equation Calculate Heat Evolved Define enthalpy and explain its classification as a state function. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. State the first law of thermodynamics. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Calculate the molar enthalpy. Calculate Heat Evolved.
From www.coursehero.com
[Solved] 5 1 point Calculate the heat evolved (KJ) for the reaction of 36.6... Course Hero Calculate Heat Evolved Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. State the first law of thermodynamics. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Define enthalpy and explain its classification as a state function. The heat evolved or. Calculate Heat Evolved.
From www.coursehero.com
[Solved] How do I calculate the Heat Evolved of a solution, using the... Course Hero Calculate Heat Evolved State the first law of thermodynamics. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Calculate the molar enthalpy of formation from combustion data using hess's law; To understand how enthalpy pertains to chemical reactions. Heat changes in chemical reactions are often measured in the laboratory under conditions in which. Calculate Heat Evolved.
From www.numerade.com
SOLVED Calculate the heat evolved (in kJ) when 27.1 g iron(III) oxide reacts accordingly. Calculate Heat Evolved State the first law of thermodynamics. Calculate the molar enthalpy of formation from combustion data using hess's law; To understand how enthalpy pertains to chemical reactions. Since h is defined by the equation:. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. This page explains hess's law, and uses it to do. Calculate Heat Evolved.
From www.youtube.com
Calculate Heat Evolved From Mass of Reactant Using Balanced Chemical Equation 004 YouTube Calculate Heat Evolved Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Since h is defined by the equation:. State the first law of thermodynamics. Define. Calculate Heat Evolved.
From www.studocu.com
Energy Thermodynamics Unit s Enthalpy Calculate the heat evolved from a reaction mixture of 13 Calculate Heat Evolved To understand how enthalpy pertains to chemical reactions. It is no more difficult than that! We have stated that the change in energy (\ (δu\)). Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Calculate the molar enthalpy of formation from combustion data using hess's law; State the first law of thermodynamics. This page explains hess's law,. Calculate Heat Evolved.
From www.chegg.com
Solved how to calculate the heat evolved, delta heat and Calculate Heat Evolved To understand how enthalpy pertains to chemical reactions. Since h is defined by the equation:. We have stated that the change in energy (\ (δu\)). Calculate the molar enthalpy of formation from combustion data using hess's law; Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere.. Calculate Heat Evolved.
From www.chegg.com
Solved Calculate the heat evolved when 75.0 mL of a 2.00M Calculate Heat Evolved Using the enthalpy of formation, calculate the unknown enthalpy of the overall. State the first law of thermodynamics. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Define enthalpy. Calculate Heat Evolved.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Calculate Heat Evolved It is no more difficult than that! This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Heat changes in chemical reactions are often measured in the laboratory. Calculate Heat Evolved.
From www.chegg.com
Solved When a solid dissolves in water, heat may be evolved Calculate Heat Evolved It is no more difficult than that! Using the enthalpy of formation, calculate the unknown enthalpy of the overall. State the first law of thermodynamics. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. The heat evolved or absorbed when a process occurs at constant pressure. Calculate Heat Evolved.
From www.numerade.com
SOLVED Calculate the heat evolved in the combutsion of ethyl alcohol Thati calculate its Calculate Heat Evolved It is no more difficult than that! Since h is defined by the equation:. Calculate the molar enthalpy of formation from combustion data using hess's law; Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. Enthalpy change is simply a measure of the amount of heat. Calculate Heat Evolved.
From www.chegg.com
Solved Calculate the amount of heat evolved when 266 g white Calculate Heat Evolved Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Define enthalpy and explain its classification as a state function. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is. Calculate Heat Evolved.
From www.youtube.com
Calculate the amount of heat evolved when (i) 500 cm^(3) of 0.1 Mhydrochloric acid is mixed with Calculate Heat Evolved To understand how enthalpy pertains to chemical reactions. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. Define enthalpy and explain its classification as a state function. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Since h is defined by the equation:. Calculate the molar enthalpy. Calculate Heat Evolved.
From www.studypool.com
SOLUTION Calculate the heat evolved Studypool Calculate Heat Evolved Since h is defined by the equation:. Define enthalpy and explain its classification as a state function. Calculate the molar enthalpy of formation from combustion data using hess's law; This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. We have stated that the change in energy. Calculate Heat Evolved.
From www.coursehero.com
[Solved] For trials 1 and 2 calculate the moles, heat evolved, heat capacity... Course Hero Calculate Heat Evolved State the first law of thermodynamics. Using the enthalpy of formation, calculate the unknown enthalpy of the overall. We have stated that the change in energy (\ (δu\)). Calculate the molar enthalpy of formation from combustion data using hess's law; Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open. Calculate Heat Evolved.
From www.chegg.com
Solved Part A Calculate the heat evolved per mole on Calculate Heat Evolved State the first law of thermodynamics. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Calculate the molar enthalpy of formation from combustion data using hess's law; To understand how enthalpy pertains to chemical reactions. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting. Calculate Heat Evolved.
From www.vrogue.co
Calculate The Amount Of Heat Evolved During The Compl vrogue.co Calculate Heat Evolved Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Define enthalpy and explain its classification as a state function. Calculate the molar enthalpy of formation from combustion data using hess's law; The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. It is. Calculate Heat Evolved.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID9481875 Calculate Heat Evolved Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. Calculate the molar enthalpy of formation from combustion data using hess's law; State the first law of thermodynamics. This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of. Calculate Heat Evolved.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calculate Heat Evolved The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Define enthalpy and explain its classification as a state function. State the first law of thermodynamics. We have stated that the change in energy (\ (δu\)). This page explains hess's law, and uses it to do some simple enthalpy change calculations. Calculate Heat Evolved.
From www.youtube.com
8.7 Enthalpy A Measure of the Heat Evolved or Absorbed YouTube Calculate Heat Evolved We have stated that the change in energy (\ (δu\)). This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. Calculate the molar enthalpy of formation from combustion data using hess's law; Define enthalpy and explain its classification as a state function. Heat changes in chemical reactions. Calculate Heat Evolved.
From spmchemistry.blog.onlinetuition.com.my
Heat of Reaction SPM Chemistry Calculate Heat Evolved The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Define enthalpy and explain its classification as a state function. Calculate the molar enthalpy of formation from combustion data using hess's law; This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of. Calculate Heat Evolved.
From www.youtube.com
Calculate the amount of heat evolved during the complete combustion of `100 ml` of liquid YouTube Calculate Heat Evolved State the first law of thermodynamics. To understand how enthalpy pertains to chemical reactions. Since h is defined by the equation:. We have stated that the change in energy (\ (δu\)). Define enthalpy and explain its classification as a state function. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is. Calculate Heat Evolved.
From slideplayer.com
PART 1 Heat and Calorimetry ppt download Calculate Heat Evolved The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. Heat changes in chemical reactions are often measured in the laboratory under conditions in which the reacting system is open to the atmosphere. We have stated that the change in energy (\ (δu\)). Calculate the molar enthalpy of formation from combustion. Calculate Heat Evolved.
From www.chegg.com
Solved Calculate the heat evolved from a reaction in which Calculate Heat Evolved Calculate the molar enthalpy of formation from combustion data using hess's law; This page explains hess's law, and uses it to do some simple enthalpy change calculations involving enthalpy changes of reaction, formation and combustion. The heat evolved or absorbed when a process occurs at constant pressure is equal to the change in enthalpy. We have stated that the change. Calculate Heat Evolved.