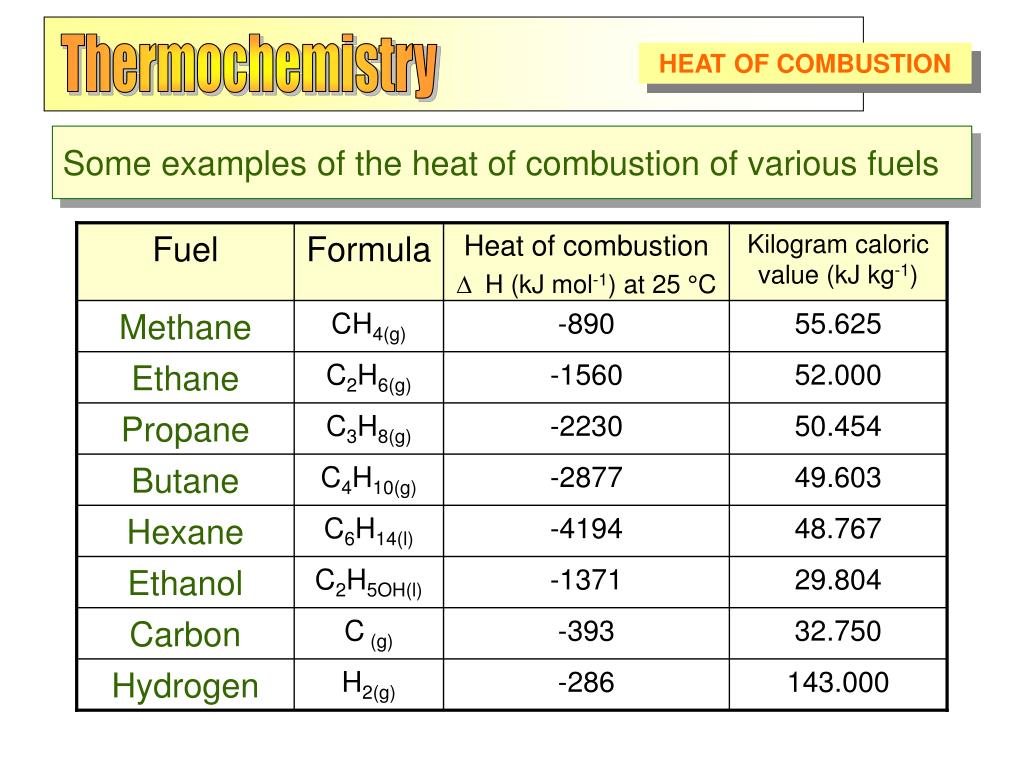
Standard Net Heat Of Combustion . The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. See heat of combustion for examples of. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard heat of combustion : The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard.
from www.slideserve.com
See heat of combustion for examples of. The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard heat of combustion : Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under.
PPT ENERGY AND REACTIONS PowerPoint Presentation, free download ID5915391
Standard Net Heat Of Combustion Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard heat of combustion : See heat of combustion for examples of. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned.
From byjus.com
Calculate standard heat of combustion of ethanol(C2H5OH(I)). Given that A,H (C2H5OH, I) 278 kJ Standard Net Heat Of Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The molar heat of combustion \ (\left ( he \right)\) is the heat. Standard Net Heat Of Combustion.
From www.numerade.com
SOLVED The standard heat of combustion of propanol, C3H7OH, is 2021 kJ/mol. How much heat would Standard Net Heat Of Combustion In thermodynamics, the term standard heat of combustion corresponds to gross heating value. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for. Standard Net Heat Of Combustion.
From www.researchgate.net
Fuel gas composition and combustion heat Download Table Standard Net Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. See heat of combustion for examples of. Standard heat of. Standard Net Heat Of Combustion.
From www.grc.nasa.gov
Combustion Standard Net Heat Of Combustion Standard heat of combustion : The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for examples. Standard Net Heat Of Combustion.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Presentation ID780619 Standard Net Heat Of Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard heat of combustion : Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The energy liberated when a substance x undergoes. Standard Net Heat Of Combustion.
From marielatinrobertson.blogspot.com
Standard Enthalpy of Combustion MarielatinRobertson Standard Net Heat Of Combustion See heat of combustion for examples of. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard heat of combustion : Standard enthalpy of combustion ($δh_c°$) is the enthalpy change. Standard Net Heat Of Combustion.
From www.pinterest.com
thermodynamics Google Search Electronic Engineering, Mechanical Engineering, Electrical Standard Net Heat Of Combustion Standard heat of combustion : Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The energy liberated when a substance x undergoes. Standard Net Heat Of Combustion.
From heerabigyan.blogspot.com
27+ Heat Of Combustion Calculation HeeraBigyan Standard Net Heat Of Combustion See heat of combustion for examples of. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard heat of combustion : Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The molar heat of combustion \ (\left ( he \right)\) is. Standard Net Heat Of Combustion.
From www.scribd.com
D4529Standard Test Method For Estimation of Net Heat of Combustion of Aviation Fuels PDF Standard Net Heat Of Combustion In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard heat of combustion : The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. See heat of combustion for examples of. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when. Standard Net Heat Of Combustion.
From www.vrogue.co
Standard Enthalpy Of Combustion vrogue.co Standard Net Heat Of Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard heat of combustion : Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of. Standard Net Heat Of Combustion.
From studylib.net
Heat of Combustion Lab Standard Net Heat Of Combustion See heat of combustion for examples of. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. Standard enthalpy of combustion (δ h c °) (δ h c. Standard Net Heat Of Combustion.
From www.chegg.com
Solved Calculate The Molar Enthalpy For The Combustion Of... Standard Net Heat Of Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. The energy liberated when a substance x undergoes complete combustion, with excess of oxygen at standard. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously. Standard Net Heat Of Combustion.
From ar.inspiredpencil.com
Enthalpy Of Combustion Table Standard Net Heat Of Combustion The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. Standard heat of combustion : Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for examples of. Standard enthalpy of combustion. Standard Net Heat Of Combustion.
From www.toppr.com
Heat of combustion of H2(g) = 241.8 kJ mol^1 C(s) = 393.5 kJ mol^1 C2H5OH(l) = 1234.7 kJ Standard Net Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for examples of. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. Standard enthalpy of combustion (δ h c °) (δ h c. Standard Net Heat Of Combustion.
From www.chegg.com
Solved Compute net heat of combustion for 3.5 moles Standard Net Heat Of Combustion Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. Standard heat of combustion : In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with.. Standard Net Heat Of Combustion.
From www.coursehero.com
[Solved] . From the data in the tables, calculate the heat of combustion... Course Hero Standard Net Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard heat of combustion : The molar heat of combustion \ (\left (. Standard Net Heat Of Combustion.
From www.wikihow.com
How to Calculate Heat of Combustion 12 Steps (with Pictures) Standard Net Heat Of Combustion Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for examples of. The molar heat of combustion \ (\left ( he \right)\) is the heat released when one mole of a substance is completely burned. Standard heat of combustion. Standard Net Heat Of Combustion.
From www.toppr.com
The standard heat of combustion of graphite carbon is 393.5 KJ mol ^1 . The standard enthalpy Standard Net Heat Of Combustion Standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. See heat of combustion for examples of. In thermodynamics, the term standard heat of combustion corresponds to gross heating value. Standard heat of combustion : The molar heat of combustion \ (\left ( he \right)\) is the heat released. Standard Net Heat Of Combustion.
From www.slideserve.com
PPT ENERGY AND REACTIONS PowerPoint Presentation, free download ID5915391 Standard Net Heat Of Combustion See heat of combustion for examples of. Standard heat of combustion : Standard enthalpy of combustion (δ h c °) (δ h c °) is the enthalpy change when 1 mole of a substance burns (combines vigorously with. Standard enthalpy of combustion ($δh_c°$) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under. The. Standard Net Heat Of Combustion.