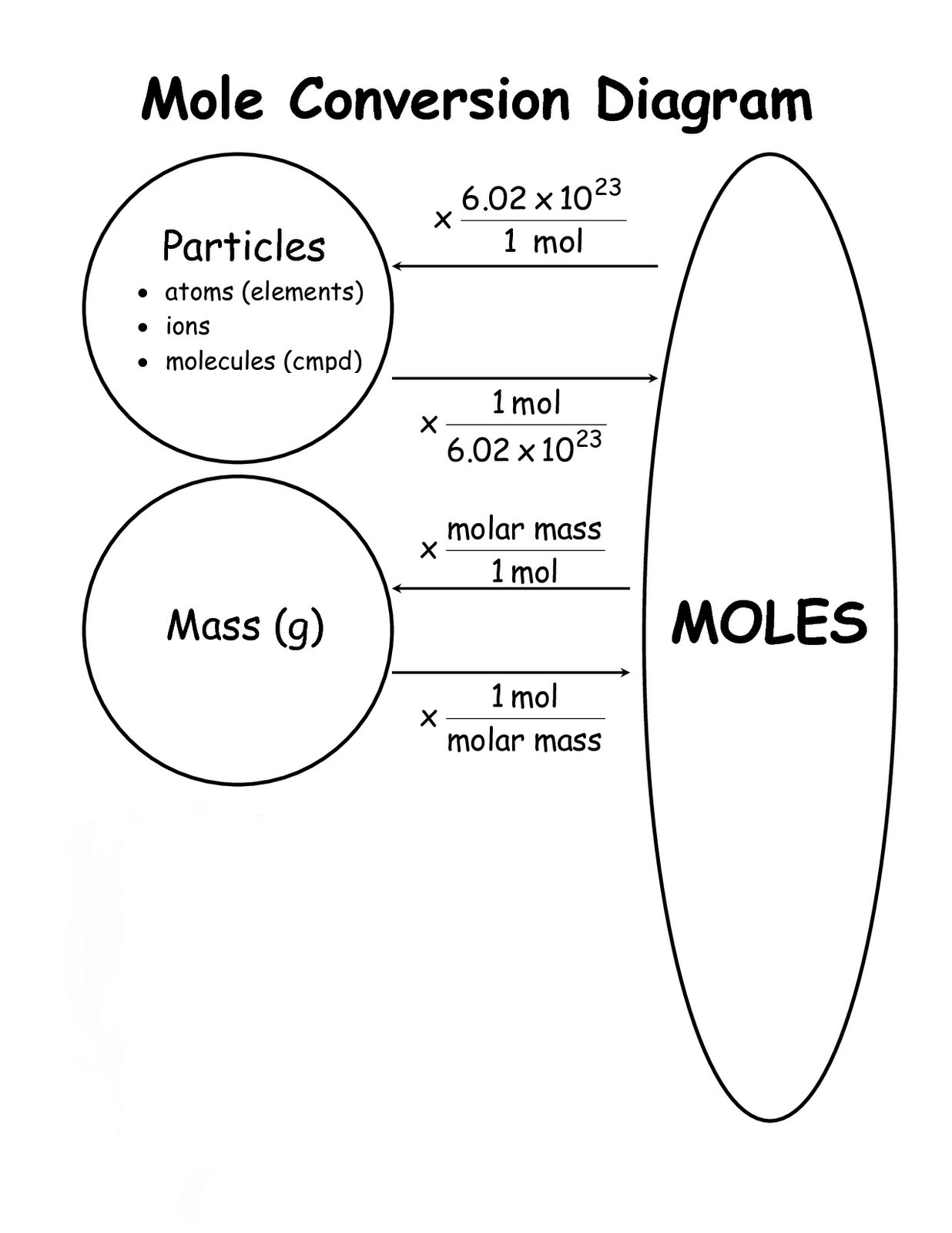
How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide . how many grams iron(iii) hydroxide in 1 mol? We assume you are converting between grams iron(iii). this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. one mole of any substance has a mass equal to its atomic or molecular weight in grams. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. you’ll need a grams to moles formula to find the moles of a substance you have. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. For example, once you have carried out a reaction, you will weigh your. For example, one mole of carbon. Grams = mole × molar mass.
from chemistrymysteries.blogspot.com
you’ll need a grams to moles formula to find the moles of a substance you have. one mole of any substance has a mass equal to its atomic or molecular weight in grams. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: For example, once you have carried out a reaction, you will weigh your. We assume you are converting between grams iron(iii). how many grams iron(iii) hydroxide in 1 mol? Grams = mole × molar mass. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. For example, one mole of carbon.
Chemistry Mysteries Mole Conversions
How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide one mole of any substance has a mass equal to its atomic or molecular weight in grams. For example, once you have carried out a reaction, you will weigh your. one mole of any substance has a mass equal to its atomic or molecular weight in grams. this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. how many grams iron(iii) hydroxide in 1 mol? to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. you’ll need a grams to moles formula to find the moles of a substance you have. For example, one mole of carbon. We assume you are converting between grams iron(iii). Grams = mole × molar mass. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass:
From es.wikihow.com
Cómo convertir gramos a moles 8 pasos (con fotos) How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, once you have carried out a reaction, you will weigh your. how many grams iron(iii) hydroxide in 1 mol? For example, one mole of carbon. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. We assume you are converting between grams iron(iii). . How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From lessonluft.z19.web.core.windows.net
Grams To Moles To Atoms Flow Chart How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. For example, once you have carried out a reaction, you will weigh your. one mole of any substance has a mass equal to its atomic or molecular weight in grams. the mass (in grams) of. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.youtube.com
Grams to Moles Conversions YouTube How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, one mole of carbon. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. Grams = mole × molar mass. you’ll need a grams to moles formula to find the moles of a substance you have. how many grams iron(iii) hydroxide in 1. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.slideserve.com
PPT Stoichiometry PowerPoint Presentation, free download ID3919556 How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide how many grams iron(iii) hydroxide in 1 mol? one mole of any substance has a mass equal to its atomic or molecular weight in grams. Grams = mole × molar mass. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. you’ll need a. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.galaxoutdoors.com
examples8 How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide We assume you are converting between grams iron(iii). to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. how many grams iron(iii) hydroxide in 1 mol? Grams = mole × molar mass. one mole of any substance has a mass equal to its atomic or. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, one mole of carbon. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. how many grams iron(iii) hydroxide in 1 mol? We assume you are converting between grams iron(iii). this grams to moles/moles to grams calculator converts between moles and grams using. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From worksheetlistmo.z21.web.core.windows.net
How To Calculate Grams From Moles How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, one mole of carbon. how many grams iron(iii) hydroxide in 1 mol? one mole of any substance has a mass equal to its atomic or molecular weight in grams. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: We assume you are converting between grams iron(iii).. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, once you have carried out a reaction, you will weigh your. one mole of any substance has a mass equal to its atomic or molecular weight in grams. We assume you are converting between grams iron(iii). how many grams iron(iii) hydroxide in 1 mol? For example, one mole of carbon. this free moles to grams. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From mavink.com
Moles Grams Atoms Conversion Chart How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide We assume you are converting between grams iron(iii). you’ll need a grams to moles formula to find the moles of a substance you have. For example, once you have carried out a reaction, you will weigh your. this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. to convert. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.numerade.com
SOLVED Given the following reaction, how many moles of iron(III) oxide How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: you’ll need a grams to moles formula to find the moles of a substance you have. We assume you are converting. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From mungfali.com
Grams To Moles Conversion Chart How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. For example, one mole of carbon. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Grams = mole × molar mass. you’ll. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles To Grams How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide one mole of any substance has a mass equal to its atomic or molecular weight in grams. Grams = mole × molar mass. how many grams iron(iii) hydroxide in 1 mol? For example, once you have carried out a reaction, you will weigh your. the mass (in grams) of a compound is equal to its molarity (in. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From peacecommission.kdsg.gov.ng
Moles Converting Between Grams And Particles How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. one mole of any substance has a mass equal to its atomic or molecular weight in grams. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass:. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From chemistrymysteries.blogspot.com
Chemistry Mysteries Mole Conversions How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, once you have carried out a reaction, you will weigh your. We assume you are converting between grams iron(iii). For example, one mole of carbon. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: to convert between moles and grams, multiply moles by the molar mass to. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From brainly.com
2. Calculate the number of moles in 0.070 g of iron(III) hydroxide How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide how many grams iron(iii) hydroxide in 1 mol? this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. For example, one mole of carbon. We assume you are converting between grams iron(iii). one mole of any substance has a mass equal to its atomic or. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.sdpuo.com
How to Find Moles in Grams A StepbyStep Guide The Cognitive Orbit How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, once you have carried out a reaction, you will weigh your. one mole of any substance has a mass equal to its atomic or molecular weight in grams. Grams = mole × molar mass. We assume you are converting between grams iron(iii). the mass (in grams) of a compound is equal to its molarity (in moles). How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From knowinsiders.com
How to Convert Grams to Moles Top Simple Steps KnowInsiders How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. We assume you are converting between grams iron(iii). Grams = mole × molar mass. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass.. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.youtube.com
Calculate the molarity of NaOH in the solution prepared by dissolving How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide Grams = mole × molar mass. For example, one mole of carbon. one mole of any substance has a mass equal to its atomic or molecular weight in grams. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: how many grams iron(iii) hydroxide in 1 mol? this. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.slideserve.com
PPT What is a MOLE? PowerPoint Presentation, free download ID5822860 How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. For example, once you have carried out a reaction, you will weigh your. For example, one mole of carbon. one mole of any substance has a mass equal to its atomic or molecular weight in grams.. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From courses.lumenlearning.com
Formula Mass and the Mole Concept General Chemistry How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Grams = mole × molar mass. For example, one mole of carbon. how many grams iron(iii) hydroxide in 1 mol? this grams to moles/moles to grams calculator converts between moles and grams using a formula. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From lessonluft.z19.web.core.windows.net
Grams To Moles To Atoms Flow Chart How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. one mole of any substance has a mass equal to its atomic or molecular weight in grams.. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles To Grams How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide We assume you are converting between grams iron(iii). For example, once you have carried out a reaction, you will weigh your. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. one mole of any substance has a mass equal to its atomic or molecular weight. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.youtube.com
Conversion of moles to gram & grams to moles YouTube How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide how many grams iron(iii) hydroxide in 1 mol? For example, one mole of carbon. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: one mole of any substance has a mass equal to its atomic or molecular weight in grams. to convert between moles and grams, multiply. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From worksheetlibhoggings.z13.web.core.windows.net
Grams To Moles Conversion Chart How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: one mole of any substance has a mass equal to its atomic or molecular weight in grams. this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. We assume you are. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.showme.com
gram to mole conversion Science, Chemistry, Stoichiometry ShowMe How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide one mole of any substance has a mass equal to its atomic or molecular weight in grams. For example, once you have carried out a reaction, you will weigh your. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. this grams to moles/moles to. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From studylib.net
Grams to Moles Worksheet How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. how many grams iron(iii) hydroxide in 1 mol? For example, once you have carried out a reaction, you will weigh your. this grams to moles/moles to grams calculator converts between moles and grams using a. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.numerade.com
SOLVED 112. Calculate the mass in grams of (a) 3.00 moles of Hg(l) (b How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide Grams = mole × molar mass. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. how many grams iron(iii) hydroxide in 1 mol? one mole of any substance has a mass equal to its atomic or molecular weight in grams. We assume you are. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.youtube.com
How to Convert Grams Fe to Moles (and Moles Fe to Atoms) YouTube How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: how many grams iron(iii) hydroxide in 1 mol? you’ll need a grams to moles formula to find the moles of a substance you have. For example, one mole of carbon. We assume you are converting between grams iron(iii). . How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From learningschoolmareggio0v.z4.web.core.windows.net
Grams To Moles Conversion Worksheets How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. one mole of any substance has a mass equal to its atomic or molecular weight in grams. you’ll need a grams to moles formula to find the moles of a substance you have. We assume. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From www.wikihow.com
How to Convert Grams to Moles 8 Steps (with Pictures) wikiHow How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, once you have carried out a reaction, you will weigh your. to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. . How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From discover.hubpages.com
Converting Among Grams, Moles, and Number The Mole Road HubPages How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: one mole of any substance has a mass equal to its atomic or molecular weight in grams.. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide For example, one mole of carbon. We assume you are converting between grams iron(iii). the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: to convert between moles and grams, multiply moles by the molar mass to get grams, or divide grams by the molar mass. this free moles. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From learningdbinventive.z14.web.core.windows.net
Calculating Moles From Grams How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. how many grams iron(iii) hydroxide in 1 mol? the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: one mole of any substance has a mass. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.
From chemistrymoleapplications.weebly.com
Converting Grams & Moles The Mole and its Applications How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide this free moles to grams calculator can instantly convert moles to grams and determine how many atoms/molecules are present in these grams. Grams = mole × molar mass. this grams to moles/moles to grams calculator converts between moles and grams using a formula of a substance. For example, once you have carried out a reaction, you will weigh. How Many Grams Are In 3 7 Moles Of Iron Iii Hydroxide.