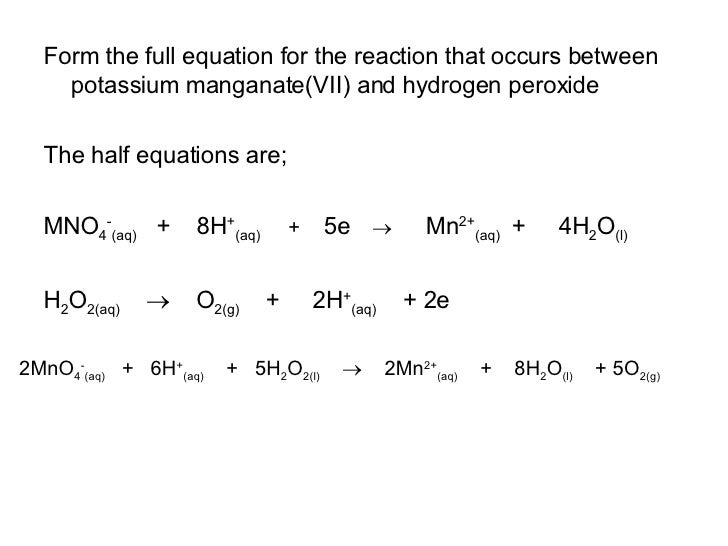
Potassium Permanganate Titration With Hydrogen Peroxide . Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. The reaction is carried out in a. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate.
from www.slideshare.net
To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide in a diluted portion of. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. The reaction is carried out in a. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with.
10 Reduction And Oxidation
Potassium Permanganate Titration With Hydrogen Peroxide This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. The reaction is carried out in a.
From studylib.net
The Potentiometric Titration of Hydrogen Peroxide Potassium Permanganate Titration With Hydrogen Peroxide This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Potassium permanganate acts as an oxidizing agent, oxidizing. The. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.instructables.com
of Hydrogen Peroxide by Potassium Permanganate Potassium Permanganate Titration With Hydrogen Peroxide The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing.. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED peroxide in solution is through redox One method for Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide in a diluted portion of. The reaction is carried out in a. This. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.reddit.com
Struggling AP student here using a titration with potassium Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. Hydrogen peroxide in a diluted portion of. This method is designed for the determination of hydrogen peroxide in. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide solution can be found by Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide in a diluted portion of. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Potassium permanganate acts as an oxidizing agent, oxidizing. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Reaction of Potassium Permanganate and Hydrogen Peroxide YouTube Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration of hydrogen peroxide by titration with. Potassium Permanganate Titration With Hydrogen Peroxide.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Potassium Permanganate Titration With Hydrogen Peroxide The reaction is carried out in a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Find the Concentration of Hydrogen Peroxide by Titration with Potassium Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. The reaction is carried out in a. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
AP Chemistry Investigation 8 Redox Titration of Hydrogen Peroxide by Potassium Permanganate Titration With Hydrogen Peroxide This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. The reaction is carried out in a.. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Potassium permanganate and hydrogen peroxide reaction YouTube Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Potassium permanganate mixed with sulfuric acid and hydrogen peroxide Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method is designed. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Chemical Experiments Hydrogen Peroxide potassium permanganate YouTube Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide in a diluted portion of. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The reaction is carried out in a. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate. Potassium Permanganate Titration With Hydrogen Peroxide.
From bulkperoxide.com
Reaction Between Hydrogen Peroxide and Potassium Permanganate Is it Potassium Permanganate Titration With Hydrogen Peroxide This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The reaction is. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Potassium Permanganate + Hydrogen Peroxide YouTube Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Potassium permanganate. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.chegg.com
Solved The concentration of hydrogen peroxide in a solution Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
of Hydrogen Peroxide Dramatic Chemical Reaction with Potassium Permanganate Titration With Hydrogen Peroxide This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. To determine the concentration of hydrogen peroxide in solution, the method. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED4) Hydrogen peroxide to water and oxygen according to Potassium Permanganate Titration With Hydrogen Peroxide This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Potassium permanganate acts as an oxidizing agent, oxidizing. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Potassium permanganate and hydrogen peroxide experiment YouTube Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide in a diluted portion of. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Potassium permanganate acts as an oxidizing agent, oxidizing. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide (h 2 o 2) is titrated. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED Assay of Hydrogen Peroxide Solution Procedure 1. Pipet 2 Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This method describes the determination of the concentration. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.coursehero.com
[Solved] A potassium permanganate solution with an approximate molarity Potassium Permanganate Titration With Hydrogen Peroxide The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. Hydrogen peroxide (h 2. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.researchgate.net
Overview of general peroxide quantitation. A.) Chemical reaction Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a diluted portion of. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. To determine the concentration of hydrogen peroxide in. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED KMnO4 (Potassium permanganate) = 4.19 (m/m) H2O2 (Hydrogen Potassium Permanganate Titration With Hydrogen Peroxide To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Potassium permanganate acts as an oxidizing agent, oxidizing. The redox titration of permanganate ions is commonly used to determine the iron. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.slideshare.net
Potassium permanganate titrations Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. The reaction is carried out in a. The redox titration of permanganate ions is commonly used to. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.chegg.com
Solved Titration of Hydrogen Peroxide Obiective In this Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide in a diluted portion of. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.youtube.com
Chemical Reaction Reaction of Potassium Permanganate With Hydrogen Potassium Permanganate Titration With Hydrogen Peroxide To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. The redox titration of permanganate ions. Potassium Permanganate Titration With Hydrogen Peroxide.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Potassium Permanganate Titration With Hydrogen Peroxide The reaction is carried out in a. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide in a diluted portion of. This method describes. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.slideshare.net
10 Reduction And Oxidation Potassium Permanganate Titration With Hydrogen Peroxide To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide content in aqueous solutions. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.chegg.com
Solved 3. Manganate(VII) ions, MnO4, oxidise hydrogen Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide in a diluted portion of. Potassium permanganate acts as an oxidizing agent, oxidizing. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The reaction is carried out in a. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.sciencephoto.com
Titration of potassium manganate (VII) Stock Image C030/3775 Potassium Permanganate Titration With Hydrogen Peroxide The reaction is carried out in a. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such. Potassium Permanganate Titration With Hydrogen Peroxide.
From solutionpharmacy.in
Assay of Hydrogen Peroxide Solution Parmacy Potassium Permanganate Titration With Hydrogen Peroxide This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. To determine the concentration of hydrogen. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Potassium Permanganate Titration With Hydrogen Peroxide This method is designed for the determination of hydrogen peroxide in aqueous solutions containing 20% to 70% hydrogen peroxide. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The redox titration of permanganate ions is commonly used to. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide solution can be found by Potassium Permanganate Titration With Hydrogen Peroxide Potassium permanganate acts as an oxidizing agent, oxidizing. The reaction is carried out in a. Hydrogen peroxide content in aqueous solutions the hydrogen peroxide content in aqueous solutions is performed by redox titration with. This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. This method is designed for the determination of hydrogen peroxide. Potassium Permanganate Titration With Hydrogen Peroxide.
From wyattctxz.blogspot.com
Reaction Between Oxalic Acid And Potassium Permanganate Equation Potassium Permanganate Titration With Hydrogen Peroxide This method describes the determination of the concentration of hydrogen peroxide by titration with potassium permanganate. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The reaction is carried out in a. Potassium permanganate acts as an oxidizing agent, oxidizing. The redox titration of permanganate ions is commonly used to determine the iron. Potassium Permanganate Titration With Hydrogen Peroxide.
From tetrahydrochlorohapiness.blogspot.com
Chemistry is your Happiness Third Lab Sesion Redox titration of Potassium Permanganate Titration With Hydrogen Peroxide The reaction is carried out in a. Hydrogen peroxide in a diluted portion of. Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method is designed for the determination of hydrogen peroxide. Potassium Permanganate Titration With Hydrogen Peroxide.
From www.pinnaxis.com
SOLVED Acidified Potassium Permanganate Solution (KMnO4), 60 OFF Potassium Permanganate Titration With Hydrogen Peroxide Hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. Hydrogen peroxide in a diluted portion of. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. This method describes the determination of the concentration of. Potassium Permanganate Titration With Hydrogen Peroxide.