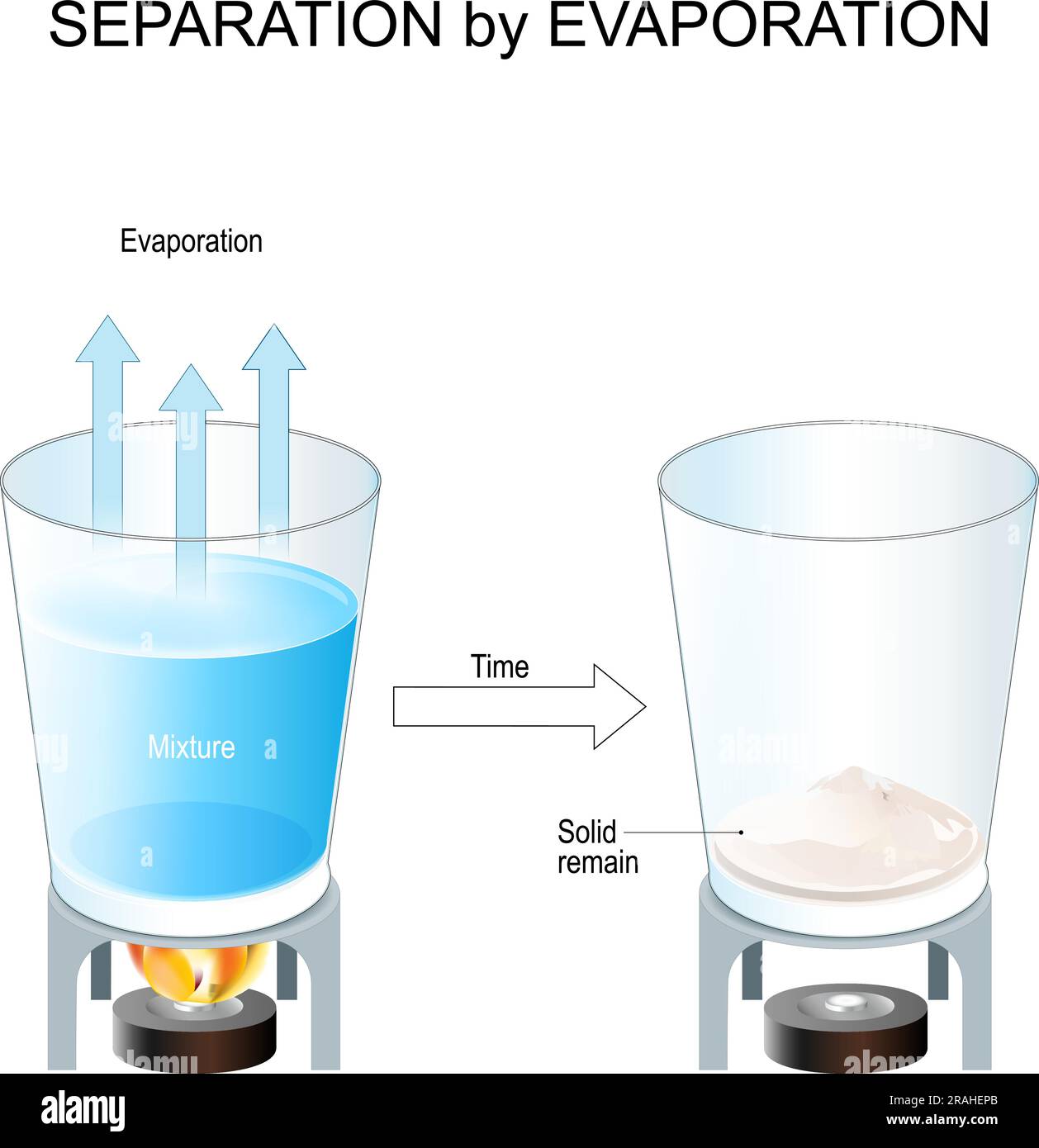
Practical Example Of Evaporation . Crystallisation is a separation technique used to obtain. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is the process where a liquid changes to a gas. Evaporation occurs when a liquid slowly turns into a gas below its boiling point.
from ar.inspiredpencil.com
Crystallisation is a separation technique used to obtain. Evaporation is the process where a liquid changes to a gas. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point.
Evaporation Chemistry
Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Crystallisation is a separation technique used to obtain. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process where a liquid changes to a gas. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications.
From www.myxxgirl.com
Separation Of Substances Class Science Chapter Evaporation And My XXX Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process where a liquid changes to a gas. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. This comprehensive guide has explored. Practical Example Of Evaporation.
From www.studocu.com
Evaporation Reference studiousguy/examplesofevaporation/ Preparing Practical Example Of Evaporation Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is the process where a liquid changes to a gas. Evaporation is a type of phase transition in which a substance directly changes. Practical Example Of Evaporation.
From www.animalia-life.club
Evaporation Examples For Kids Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. It happens when single particles at the surface of a liquid have enough. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Evaporation Chemistry Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is the process where a liquid changes to a gas. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Crystallisation is a separation technique used to obtain. Evaporation is. Practical Example Of Evaporation.
From edu.rsc.org
Evaporation explained Infographic RSC Education Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Crystallisation is a separation technique used to obtain. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is described as the phase in which the water state takes place. Practical Example Of Evaporation.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Practical Example Of Evaporation Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is described as. Practical Example Of Evaporation.
From quizizz.com
Evaporation 2K plays Quizizz Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is a. Practical Example Of Evaporation.
From mavink.com
Labelled Diagram Of Evaporation Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering. Practical Example Of Evaporation.
From loeagzbbe.blob.core.windows.net
Evaporation Model Process at Scott Williams blog Practical Example Of Evaporation Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. It happens when single particles at the surface. Practical Example Of Evaporation.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase in which. Practical Example Of Evaporation.
From www.dreamstime.com
Vector Schematic Representation of the Water Evaporation Process in Practical Example Of Evaporation Evaporation is the process where a liquid changes to a gas. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase in which the water. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Evaporation Examples Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the process. Practical Example Of Evaporation.
From www.youtube.com
Evaporation Experiment YouTube Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. This comprehensive guide has. Practical Example Of Evaporation.
From www.youtube.com
What is Evaporation Meaning Is Evaporation a Cooling process Practical Example Of Evaporation Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is the. Practical Example Of Evaporation.
From mavink.com
What Is Evaporation For Kids Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Crystallisation is a separation technique used to obtain. Evaporation is the process where a liquid changes to a gas. Evaporation is a. Practical Example Of Evaporation.
From chemistnotes.com
Evaporation Definition, Process, and 5 Reliable Example Chemistry Notes Practical Example Of Evaporation Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Crystallisation is a separation technique used to obtain. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation occurs when a liquid slowly turns into a. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Crystallisation Method Of Separating Mixtures Practical Example Of Evaporation Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. It happens when single particles at the surface of a liquid have enough energy to break away from the other. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Evaporation Examples Practical Example Of Evaporation Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is the process where a liquid changes to a gas. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Crystallisation is a separation technique used to obtain. Evaporation occurs when a liquid slowly. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Evaporation Chemistry Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. This comprehensive guide has. Practical Example Of Evaporation.
From education-portal.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation Practical Example Of Evaporation Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. It happens when single particles at the. Practical Example Of Evaporation.
From dxonbzsdj.blob.core.windows.net
Water Cycle Meaning Of Deposition at Nathan Johnson blog Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. It happens when. Practical Example Of Evaporation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Practical Example Of Evaporation Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Crystallisation is a separation technique used to obtain. It happens when single particles at the surface of a liquid have enough energy to break. Practical Example Of Evaporation.
From www.researchgate.net
(a) Schematics of the solvent evaporation crystallization method. (b Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. It happens when single particles at. Practical Example Of Evaporation.
From www.youtube.com
What is evaporation How salt is made Evaporation process & facts Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is described as the phase in which the water state takes place from the liquid to. Practical Example Of Evaporation.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts Practical Example Of Evaporation Evaporation is the process where a liquid changes to a gas. Crystallisation is a separation technique used to obtain. Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. This comprehensive. Practical Example Of Evaporation.
From www.youtube.com
what is evaporation and factor effect of evaporation class 9 we YouTube Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is the process where a liquid changes to a gas. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. Evaporation is described as the phase. Practical Example Of Evaporation.
From www.animalia-life.club
Evaporation Examples For Kids Practical Example Of Evaporation Evaporation is described as the phase in which the water state takes place from the liquid to the gaseous or vapour state. Evaporation occurs when a liquid slowly turns into a gas below its boiling point. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Crystallisation is a separation technique used. Practical Example Of Evaporation.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Practical Example Of Evaporation Evaporation occurs when a liquid slowly turns into a gas below its boiling point. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is described as the phase in. Practical Example Of Evaporation.
From www.youtube.com
What is Evaporation Science for Kids YouTube Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is the process where a liquid changes to a gas. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Crystallisation is a separation technique used to obtain. Evaporation is. Practical Example Of Evaporation.
From www.animalia-life.club
Evaporation Process Of Separating Mixtures Practical Example Of Evaporation Evaporation is the process where a liquid changes to a gas. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation is described as the phase in which the water. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Evaporation Examples Practical Example Of Evaporation Crystallisation is a separation technique used to obtain. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is the process where a liquid changes to a gas. Evaporation is. Practical Example Of Evaporation.
From www.dreamstime.com
Diagram Showing Evaporation Separating Mixtures Stock Vector Practical Example Of Evaporation This comprehensive guide has explored the driving forces behind evaporation, the key factors influencing the rate of evaporation,. Evaporation is the process where a liquid changes to a gas. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. It happens when single particles at the surface of a liquid have enough energy. Practical Example Of Evaporation.
From www.bbc.co.uk
Evaporation BBC Bitesize Practical Example Of Evaporation Evaporation occurs when a liquid slowly turns into a gas below its boiling point. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation is the process where a liquid changes to a gas. Evaporation is a fundamental physical process that plays a crucial role in various scientific. Practical Example Of Evaporation.
From www.pinterest.com
Evaporation Evaporation, Basic, Techniques Practical Example Of Evaporation Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation is a fundamental physical process that plays a crucial role in various scientific and engineering applications. It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation. Practical Example Of Evaporation.
From ar.inspiredpencil.com
Examples Of Evaporation For Kids Practical Example Of Evaporation It happens when single particles at the surface of a liquid have enough energy to break away from the other particles. Evaporation is the process where a liquid changes to a gas. Evaporation is a type of phase transition in which a substance directly changes from a liquid state to its gaseous state. Evaporation is a fundamental physical process that. Practical Example Of Evaporation.