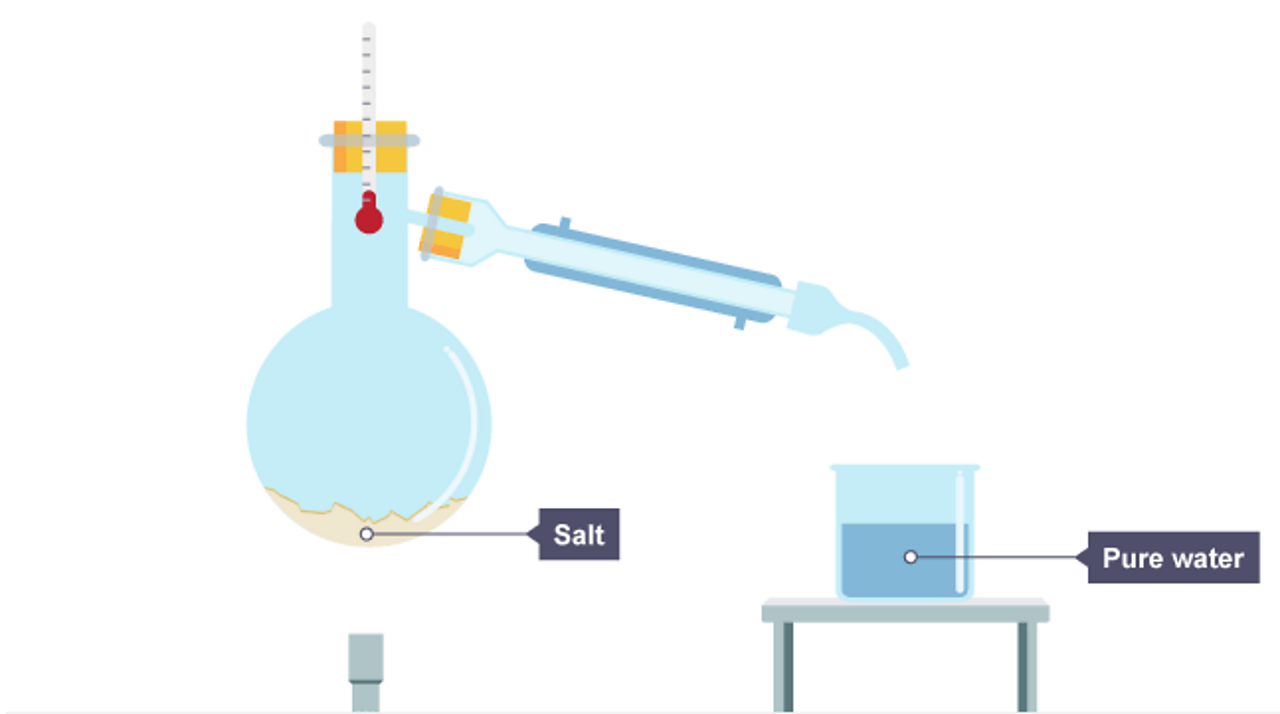
Salt In Water Chemistry . Seawater is a complex mixture of 96.5 percent water, 2.5 percent. In chemistry, it results in a solution, as the ionic bond of nacl. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. The temperature needed to boil will increase by about 0.5 c for every 58 grams of.
from www.bbc.co.uk
If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater is a complex mixture of 96.5 percent water, 2.5 percent.
Distillation BBC Bitesize
Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. In chemistry, it results in a solution, as the ionic bond of nacl. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans.
From www.bbc.co.uk
Distillation BBC Bitesize Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. The temperature needed to boil will increase. Salt In Water Chemistry.
From saltwaterpoolreport.com
Saltwater Pool Chemistry (Ideal Levels Chart 101) Salt Water Pool Report Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive. Salt In Water Chemistry.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. Seawater, water that. Salt In Water Chemistry.
From guidelibefficience.z13.web.core.windows.net
A Diagram Of A Solution Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At the molecular. Salt In Water Chemistry.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. If you add salt to water, you raise the boiling point, or the temperature at which water boils. In chemistry, it results in a solution, as the ionic bond of nacl. The temperature needed to boil will increase. Salt In Water Chemistry.
From makeup.vidalondon.net
Chemical Makeup Of Seawater Makeup Vidalondon Salt In Water Chemistry Seawater is a complex mixture of 96.5 percent water, 2.5 percent. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. In chemistry, it results in a solution, as the ionic bond of nacl. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Salt dissolved. Salt In Water Chemistry.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. At the molecular level, salt dissolves in water due to electrical charges and due to. Salt In Water Chemistry.
From www.youtube.com
Salt Water Density Experiment Daycare Activities YouTube Salt In Water Chemistry In chemistry, it results in a solution, as the ionic bond of nacl. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges.. Salt In Water Chemistry.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. At the molecular level, salt dissolves in water due to electrical charges and due to. Salt In Water Chemistry.
From www.thewildwaycoffee.com
Off Kilter // Coffee Blog The Wild Way Coffee Creations Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. If you add salt to water, you raise. Salt In Water Chemistry.
From www.youtube.com
Saltwater Solution Experiment—Part 1 Chemistry The Good and the Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater. Salt In Water Chemistry.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. The temperature needed to boil will. Salt In Water Chemistry.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. In chemistry,. Salt In Water Chemistry.
From tammiestime.blogspot.co.uk
Walking by the Way April 2011 Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater is a. Salt In Water Chemistry.
From techiescientist.com
Is Saltwater a Homogeneous or a Heterogeneous Mixture? Techiescientist Salt In Water Chemistry Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. If you add salt to water, you raise the boiling point, or the temperature at which water boils. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. At. Salt In Water Chemistry.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. In chemistry, it results in a solution, as the ionic bond of nacl. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At. Salt In Water Chemistry.
From www.hpacmag.com
fig6bsaltmoleculesinH2O HPAC Magazine Salt In Water Chemistry Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. Salt dissolved in water is a rough description of earth's oceans. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. At the molecular level, salt dissolves in water due to electrical charges and due to the. Salt In Water Chemistry.
From studylib.net
salt water chemistry Salt In Water Chemistry Seawater is a complex mixture of 96.5 percent water, 2.5 percent. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater, water that. Salt In Water Chemistry.
From electrimetha.blogspot.com
Electrilike เซลล์ไฟฟ้าเคมีจากน้ำเค็ม Salt Water Battery Salt In Water Chemistry In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the. Salt In Water Chemistry.
From chemistry.stackexchange.com
chemistry Why do we need salt in a salt water battery Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. In chemistry, it results in a solution, as the ionic bond of nacl. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the. Salt In Water Chemistry.
From www.walmart.com
Natural Chemistry Swimming Pool Salt Water Magic Closing Chemical Kit Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. If you add salt to water,. Salt In Water Chemistry.
From worksheetfullplaza.z21.web.core.windows.net
Salt Water Pool Chemistry Chart Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. In chemistry, it results in a solution, as the ionic bond of nacl. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. At. Salt In Water Chemistry.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. In chemistry, it results in a solution, as the ionic bond of nacl. Salt dissolved in water is a rough description of earth's oceans. If you add. Salt In Water Chemistry.
From mirjamglessmer.com
sea water Archives Adventures in Oceanography and Teaching Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Seawater, water that. Salt In Water Chemistry.
From www.shutterstock.com
Chemical Reaction Water Salt Stock Vector (Royalty Free) 165176114 Salt In Water Chemistry At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it. Salt In Water Chemistry.
From www.zagerspoolspa.com
Pool Chemistry Cheat Sheet for Chlorine Pools Zagers Pool & Spa Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which. Salt In Water Chemistry.
From saltwaterpoolreport.com
Salt Water Pool Chemicals For Dummies Salt Water Pool Report Salt In Water Chemistry Seawater, water that makes up the oceans and seas, covering more than 70 percent of earth’s surface. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. If you add salt to water, you raise the boiling point, or the temperature at which water boils. In chemistry, it results in a solution, as the ionic bond of nacl. The. Salt In Water Chemistry.
From www.saubhaya.com
Chemical Makeup Of Salt Water Saubhaya Makeup Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with. Salt In Water Chemistry.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater, water that makes up the oceans and seas, covering more than 70 percent of. Salt In Water Chemistry.
From abigailsaunders.z13.web.core.windows.net
Pool Water Chemistry Chart Salt In Water Chemistry The temperature needed to boil will increase by about 0.5 c for every 58 grams of. In chemistry, it results in a solution, as the ionic bond of nacl. If you add salt to water, you raise the boiling point, or the temperature at which water boils. At the molecular level, salt dissolves in water due to electrical charges and. Salt In Water Chemistry.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Salt In Water Chemistry Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater is a. Salt In Water Chemistry.
From leisurepoolscanada.ca
Pool Maintenance 101 Water Chemistry Leisure Pools Canada Salt In Water Chemistry In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Salt. Salt In Water Chemistry.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? in Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. Salt dissolved in water is a rough description of earth's oceans. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Seawater, water that makes up the oceans and seas, covering more than 70 percent of. Salt In Water Chemistry.
From aquanerd.com
Water chemistry 101 Top water parameters to test for in saltwater Salt In Water Chemistry In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges. Salt dissolved in water is a rough description of earth's oceans. Seawater, water that makes up the. Salt In Water Chemistry.
From www.shutterstock.com
357 Iron Salt Chemistry Images, Stock Photos & Vectors Shutterstock Salt In Water Chemistry If you add salt to water, you raise the boiling point, or the temperature at which water boils. Seawater is a complex mixture of 96.5 percent water, 2.5 percent. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in. Salt In Water Chemistry.