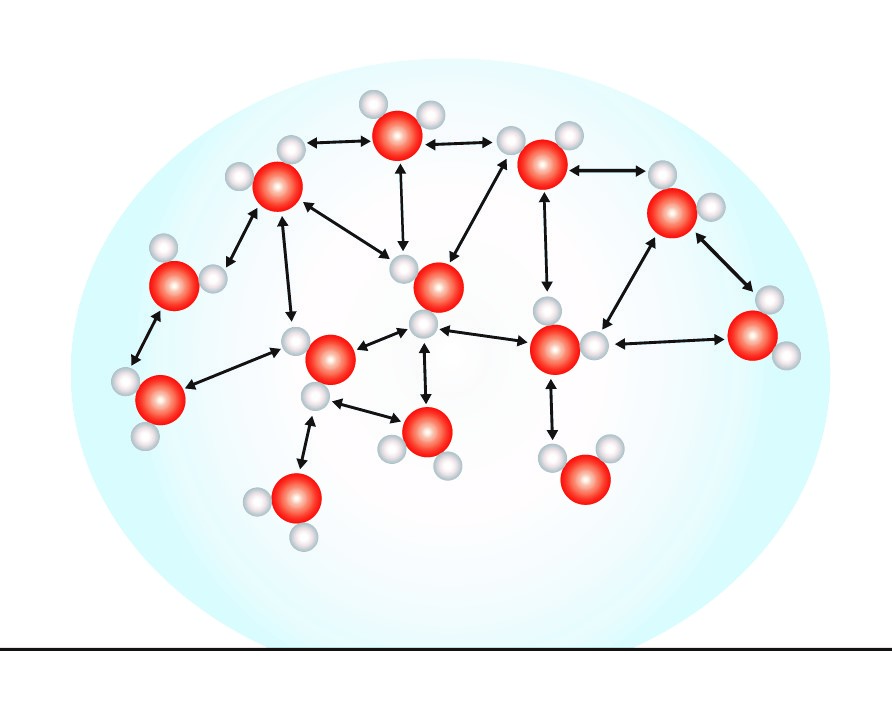
How To Determine Surface Tension Of A Molecule . The surface tension of a liquid results from an imbalance of intermolecular. Here, the water molecules in the droplet are. • surface tension is a property of a liquid that allows them to resist external forces. However, molecules at the surface are pulled downwards and sideways by other liquid. The chemical characteristics that determine how high the. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Molecules within a liquid are pulled equally in all directions by intermolecular forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the energy required to increase the surface area of a liquid by a given amount. It combines the concepts of cohesion and adhesion. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance.
from www.acs.org
Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. However, molecules at the surface are pulled downwards and sideways by other liquid. • surface tension is a property of a liquid that allows them to resist external forces. The chemical characteristics that determine how high the. The surface tension of a liquid results from an imbalance of intermolecular. Here, the water molecules in the droplet are. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance.
Simulations & Videos for Lesson 5.2 Surface Tension American
How To Determine Surface Tension Of A Molecule It combines the concepts of cohesion and adhesion. It combines the concepts of cohesion and adhesion. However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The chemical characteristics that determine how high the. Here, the water molecules in the droplet are. The surface tension of a liquid results from an imbalance of intermolecular. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Molecules within a liquid are pulled equally in all directions by intermolecular forces. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. The stronger the intermolecular interactions, the.
From pressbooks.online.ucf.edu
11.2 Properties of Liquids Chemistry Fundamentals How To Determine Surface Tension Of A Molecule Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. The surface tension of a liquid results from an imbalance of intermolecular. It combines the concepts of cohesion and adhesion. Here, the water molecules in the droplet are. Surface tension is the force that holds atoms or molecules. How To Determine Surface Tension Of A Molecule.
From www.remet.com
Surface Tension The missing link to a good prime coat? REMET How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. • surface tension is a property of a liquid that allows them to resist external forces. The stronger the intermolecular interactions, the. Here, the water molecules in the droplet are. Surface tension is the force that holds atoms or. How To Determine Surface Tension Of A Molecule.
From www.slideserve.com
PPT Chemistry 231 PowerPoint Presentation, free download ID1276788 How To Determine Surface Tension Of A Molecule However, molecules at the surface are pulled downwards and sideways by other liquid. The stronger the intermolecular interactions, the. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. It combines. How To Determine Surface Tension Of A Molecule.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How To Determine Surface Tension Of A Molecule Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Molecules within a liquid are pulled equally in all directions by intermolecular forces. • surface tension is a property of a liquid that allows them to resist external forces. Here, the water molecules in the droplet are. Surface. How To Determine Surface Tension Of A Molecule.
From www.youtube.com
Surface Tension Molecular Theory of Surface Tension Class 11 unit How To Determine Surface Tension Of A Molecule The chemical characteristics that determine how high the. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. It combines the concepts of cohesion and adhesion. Molecules. How To Determine Surface Tension Of A Molecule.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How To Determine Surface Tension Of A Molecule Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Here, the water molecules in the droplet are. The surface tension of a liquid results from an imbalance of intermolecular. The stronger the intermolecular interactions, the. Molecules within a liquid are pulled equally in all directions by. How To Determine Surface Tension Of A Molecule.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry How To Determine Surface Tension Of A Molecule Here, the water molecules in the droplet are. However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. The chemical characteristics that determine how high the. Surface tension is the energy, or work, required. How To Determine Surface Tension Of A Molecule.
From www.biolinscientific.com
3 ways to measure surface tension How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the. How To Determine Surface Tension Of A Molecule.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How To Determine Surface Tension Of A Molecule Here, the water molecules in the droplet are. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. • surface tension is a property of a liquid that allows them to resist external forces. It combines the concepts of cohesion and adhesion. Surface tension is the energy required to increase. How To Determine Surface Tension Of A Molecule.
From www.animalia-life.club
Surface Tension Of Water On A Leaf How To Determine Surface Tension Of A Molecule The stronger the intermolecular interactions, the. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Surface tension is the energy required to increase the surface area of a liquid by a given amount. However, molecules at the surface are pulled downwards and sideways by other liquid.. How To Determine Surface Tension Of A Molecule.
From www.researchgate.net
Surface tension decreases with increasing the surfactant concentration How To Determine Surface Tension Of A Molecule However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension of a liquid. How To Determine Surface Tension Of A Molecule.
From wedophysics.blogspot.com
Surface tension (Fluid statics) Complete notes Important questions How To Determine Surface Tension Of A Molecule However, molecules at the surface are pulled downwards and sideways by other liquid. Molecules within a liquid are pulled equally in all directions by intermolecular forces. It combines the concepts of cohesion and adhesion. The surface tension of a liquid results from an imbalance of intermolecular. Here, the water molecules in the droplet are. Surface tension is the energy required. How To Determine Surface Tension Of A Molecule.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How To Determine Surface Tension Of A Molecule • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another. How To Determine Surface Tension Of A Molecule.
From www.biolinscientific.com
What is surface free energy? How To Determine Surface Tension Of A Molecule Here, the water molecules in the droplet are. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. The chemical characteristics that determine how high the. • surface tension is a property of a liquid that allows them to resist external forces. Molecules within a liquid are pulled. How To Determine Surface Tension Of A Molecule.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint How To Determine Surface Tension Of A Molecule However, molecules at the surface are pulled downwards and sideways by other liquid. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area. How To Determine Surface Tension Of A Molecule.
From chem.libretexts.org
11.3 Some Properties of Liquids Chemistry LibreTexts How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Here, the water molecules in the droplet are. The surface tension of a liquid results from an imbalance of intermolecular. Molecules within a liquid are pulled equally in all directions by intermolecular forces. Surface tension is the energy required. How To Determine Surface Tension Of A Molecule.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American How To Determine Surface Tension Of A Molecule Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Molecules within a liquid are pulled equally in all directions by intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is a measurable value that tells us. How To Determine Surface Tension Of A Molecule.
From readchemistry.com
Determination of Surface Tension Read Chemistry How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is. How To Determine Surface Tension Of A Molecule.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. It combines the concepts of cohesion and adhesion. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. The surface tension of a liquid results from. How To Determine Surface Tension Of A Molecule.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How To Determine Surface Tension Of A Molecule The surface tension of a liquid results from an imbalance of intermolecular. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. • surface tension is a property of a liquid that allows them to resist external forces. The chemical characteristics that determine how high the. The. How To Determine Surface Tension Of A Molecule.
From www.chegg.com
Solved Which molecule has the lowest surface tension? How To Determine Surface Tension Of A Molecule Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. However, molecules at the surface are pulled downwards and sideways by other liquid. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the force that holds atoms or. How To Determine Surface Tension Of A Molecule.
From www.numerade.com
SOLVED (c) To help explain surface tension, use single water molecule How To Determine Surface Tension Of A Molecule • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. The stronger the intermolecular interactions, the. The chemical characteristics that determine how high the. The surface tension of a liquid results from. How To Determine Surface Tension Of A Molecule.
From www.youtube.com
Molecular Theory Of Surface Tension (Hindi) Class 11 Physics YouTube How To Determine Surface Tension Of A Molecule The chemical characteristics that determine how high the. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. However, molecules at the surface are pulled downwards and sideways by other liquid. The. How To Determine Surface Tension Of A Molecule.
From byjus.com
Explain the surface tension phenomenon with examples. How To Determine Surface Tension Of A Molecule Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. However, molecules at the surface are pulled downwards and sideways by other liquid. The stronger the intermolecular interactions, the. Surface tension is the force that holds atoms or molecules of the same substance together when they are in. How To Determine Surface Tension Of A Molecule.
From www.youtube.com
2.4.1 Molecular Theory of Surface Tension YouTube How To Determine Surface Tension Of A Molecule Here, the water molecules in the droplet are. It combines the concepts of cohesion and adhesion. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid. How To Determine Surface Tension Of A Molecule.
From byjus.com
Explain surface tension on the basis of molecular theory. How To Determine Surface Tension Of A Molecule Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The. How To Determine Surface Tension Of A Molecule.
From universe-review.ca
Molecules How To Determine Surface Tension Of A Molecule It combines the concepts of cohesion and adhesion. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the. Surface tension is the force that holds atoms. How To Determine Surface Tension Of A Molecule.
From recemev.jimdo.com
Surface Tension recemev How To Determine Surface Tension Of A Molecule It combines the concepts of cohesion and adhesion. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Molecules within a liquid are pulled equally in all directions by intermolecular forces. However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is. How To Determine Surface Tension Of A Molecule.
From studylib.net
Intermolecular Forces and Solubility How To Determine Surface Tension Of A Molecule Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Molecules within a liquid are pulled. How To Determine Surface Tension Of A Molecule.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube How To Determine Surface Tension Of A Molecule • surface tension is a property of a liquid that allows them to resist external forces. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension. How To Determine Surface Tension Of A Molecule.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How To Determine Surface Tension Of A Molecule Here, the water molecules in the droplet are. However, molecules at the surface are pulled downwards and sideways by other liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the force that holds atoms or molecules of the same substance together when they are in. How To Determine Surface Tension Of A Molecule.
From www.vrogue.co
To Determine The Surface Tension Of Given Liquid Usin vrogue.co How To Determine Surface Tension Of A Molecule The stronger the intermolecular interactions, the. The surface tension of a liquid results from an imbalance of intermolecular. Molecules within a liquid are pulled equally in all directions by intermolecular forces. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the force that holds atoms. How To Determine Surface Tension Of A Molecule.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How To Determine Surface Tension Of A Molecule Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Here, the water molecules in the droplet are. The chemical characteristics that determine how high the. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area.. How To Determine Surface Tension Of A Molecule.
From brainly.in
1. Define surface tension. Write formula, unit and dimension of surface How To Determine Surface Tension Of A Molecule Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Here, the water molecules in the droplet are. The surface tension of a liquid results. How To Determine Surface Tension Of A Molecule.
From www.pinterest.com
Surface tension, Surface, Tension How To Determine Surface Tension Of A Molecule It combines the concepts of cohesion and adhesion. The surface tension of a liquid results from an imbalance of intermolecular. Surface tension is a measurable value that tells us how strong the attraction between molecules on the surface of a liquid is. However, molecules at the surface are pulled downwards and sideways by other liquid. The stronger the intermolecular interactions,. How To Determine Surface Tension Of A Molecule.