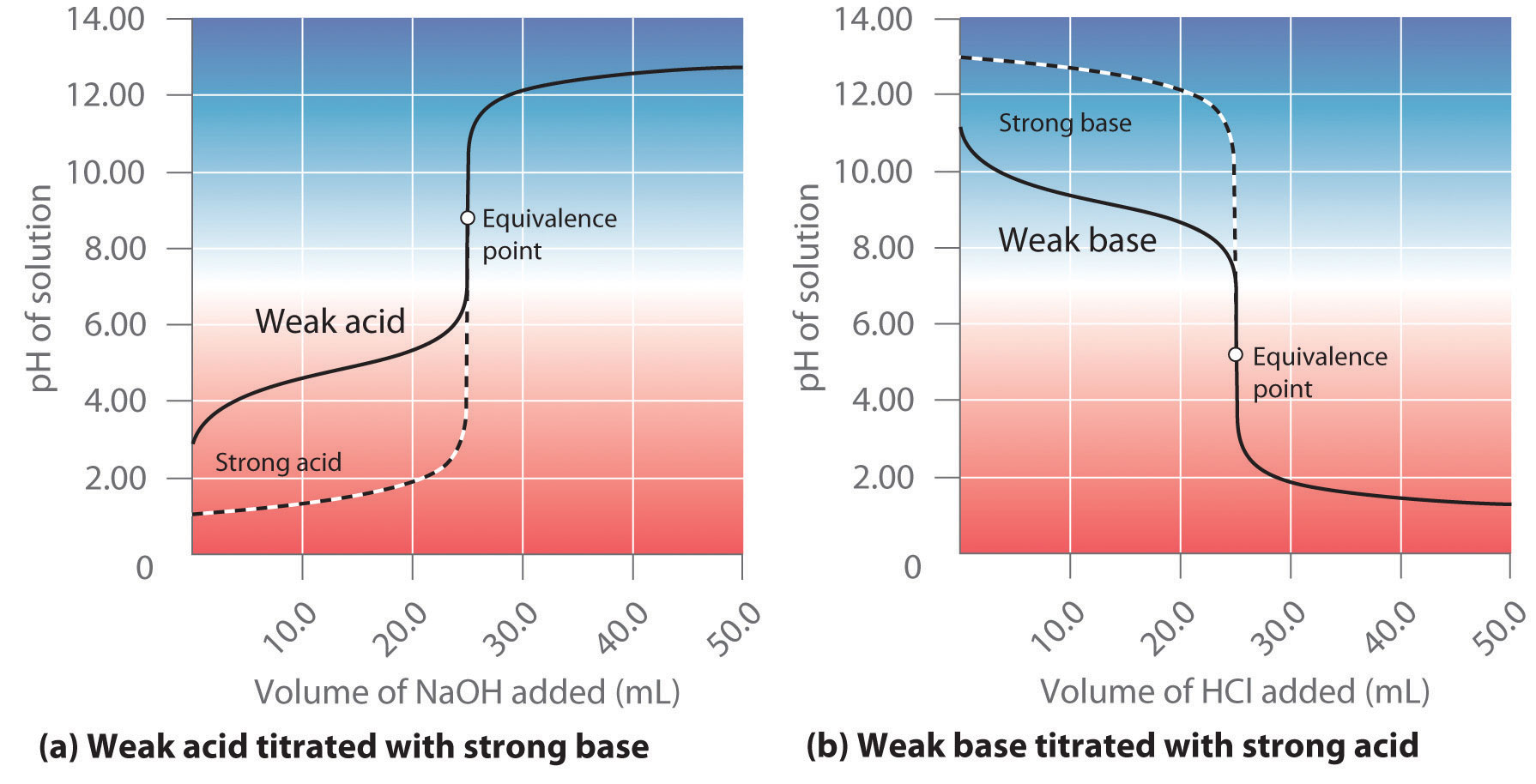
What Is An End Point In Titration . In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The endpoint refers to the point in the experiment. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The red arrow shows the location of the titration’s end point. The end point is where the titration ends in practice. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The end point typically comes. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. Endpoint and equivalence point are two terms commonly used in titration experiments. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of.
from chem.libretexts.org
Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point.
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts
What Is An End Point In Titration The red arrow shows the location of the titration’s end point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. The red arrow shows the location of the titration’s end point. The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The endpoint refers to the point in the experiment. The end point typically comes. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Endpoint and equivalence point are two terms commonly used in titration experiments.
From www.animalia-life.club
Endpoint Titration What Is An End Point In Titration The end point typically comes. Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Another method for locating. What Is An End Point In Titration.
From philschatz.com
AcidBase Titrations · Chemistry What Is An End Point In Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a titration refers to. What Is An End Point In Titration.
From proper-cooking.info
Endpoint Titration What Is An End Point In Titration Endpoint and equivalence point are two terms commonly used in titration experiments. The end point typically comes. The endpoint refers to the point in the experiment. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The end point is where the. What Is An End Point In Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts What Is An End Point In Titration The end point typically comes. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. The endpoint refers to the point in the experiment. The red arrow shows the location of the titration’s end point. A titration is a volumetric technique in which a solution of one reactant (the titrant). What Is An End Point In Titration.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube What Is An End Point In Titration The end point typically comes. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The end point is where the titration ends in practice. The red arrow shows the location. What Is An End Point In Titration.
From hxenjakty.blob.core.windows.net
Titration Types Definition at John Justice blog What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Using a ph probe and using a colour indicator. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. The endpoint refers to the point in the. What Is An End Point In Titration.
From ar.inspiredpencil.com
Titration Curve Labeled What Is An End Point In Titration The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. What Is An End Point In Titration.
From www.animalia-life.club
Endpoint Titration What Is An End Point In Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Endpoint and equivalence point are two terms commonly used in titration experiments. A titration is a volumetric technique in which a solution. What Is An End Point In Titration.
From www.learnsci.com
LearnSci LabSim Titration End Point What Is An End Point In Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. The closer the end point to the equivalence point the better, but it is often not easy to find a. What Is An End Point In Titration.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers What Is An End Point In Titration Using a ph probe and using a colour indicator. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are. What Is An End Point In Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki What Is An End Point In Titration The endpoint refers to the point in the experiment. The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. The red arrow shows the location of the titration’s end point. A. What Is An End Point In Titration.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph What Is An End Point In Titration Using a ph probe and using a colour indicator. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The end point typically comes. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter we noted that a titration’s. What Is An End Point In Titration.
From www.slideserve.com
PPT CH 103 ACIDBASE TITRATIONS PowerPoint Presentation, free What Is An End Point In Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The endpoint refers to the point in the experiment.. What Is An End Point In Titration.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the. What Is An End Point In Titration.
From hxeetojpk.blob.core.windows.net
Sharp End Point Titration at Tyrone McKenna blog What Is An End Point In Titration The end point is where the titration ends in practice. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter. What Is An End Point In Titration.
From www.animalia-life.club
Endpoint Titration What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The endpoint refers to the point in the experiment. The end point typically comes. The closer the end. What Is An End Point In Titration.
From exoafzput.blob.core.windows.net
Titration Graph Point at Arleen McKoy blog What Is An End Point In Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration. What Is An End Point In Titration.
From ar.inspiredpencil.com
Titration Phenolphthalein What Is An End Point In Titration For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Endpoint and equivalence point are two terms commonly used in titration experiments. Using a ph probe and using a colour indicator. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The. What Is An End Point In Titration.
From www.esds.co.in
What is an Endpoint & How Endpoint Security Works ESDS What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The end point is where the titration ends in practice. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The end point typically. What Is An End Point In Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during What Is An End Point In Titration The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The end point typically comes. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The red arrow shows the location of the titration’s end point.. What Is An End Point In Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. The red arrow. What Is An End Point In Titration.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder What Is An End Point In Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator. The end point typically comes. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint. What Is An End Point In Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME What Is An End Point In Titration The end point typically comes. The red arrow shows the location of the titration’s end point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such. What Is An End Point In Titration.
From www.animalia-life.club
Endpoint Titration What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Another method for locating the. What Is An End Point In Titration.
From www.youtube.com
Titration end point using phenolphthalein indicator YouTube What Is An End Point In Titration Endpoint and equivalence point are two terms commonly used in titration experiments. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. What Is An End Point In Titration.
From www.alamy.com
Titration hires stock photography and images Alamy What Is An End Point In Titration Using a ph probe and using a colour indicator. Endpoint and equivalence point are two terms commonly used in titration experiments. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The completion of a titration is the end point, detected by some type of physical change. What Is An End Point In Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download What Is An End Point In Titration Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. A titration is a volumetric technique in which a solution. What Is An End Point In Titration.
From www.differencebetween.com
Difference Between Endpoint and Stoichiometric Point Compare the What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the. What Is An End Point In Titration.
From www.animalia-life.club
Endpoint Titration What Is An End Point In Titration The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The end point is where the titration ends in practice. Using a ph probe and using a colour indicator. A titration is a volumetric technique in which a solution of one reactant (the titrant) is. What Is An End Point In Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts What Is An End Point In Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The end point typically comes. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. For a ph titration (between an acid and a base), there. What Is An End Point In Titration.
From www.vrogue.co
Strong Acid Weak Base Titrations vrogue.co What Is An End Point In Titration Endpoint and equivalence point are two terms commonly used in titration experiments. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. The end point is where the titration ends in practice. The endpoint refers to. What Is An End Point In Titration.
From www.slideserve.com
PPT Chapter 8 Acids and Bases PowerPoint Presentation, free download What Is An End Point In Titration Using a ph probe and using a colour indicator. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The red arrow shows the location of the titration’s. What Is An End Point In Titration.
From exovodadv.blob.core.windows.net
Titration End Point Meaning at Jenny Ingram blog What Is An End Point In Titration Endpoint and equivalence point are two terms commonly used in titration experiments. The end point is where the titration ends in practice. The end point typically comes. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The. What Is An End Point In Titration.
From www.spiceworks.com
What Is Endpoint Security? Definition, Key Components, and Best What Is An End Point In Titration The end point typically comes. The red arrow shows the location of the titration’s end point. Endpoint and equivalence point are two terms commonly used in titration experiments. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The endpoint refers to the point in the experiment. Using a ph probe. What Is An End Point In Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts What Is An End Point In Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. What Is An End Point In Titration.